Abstract
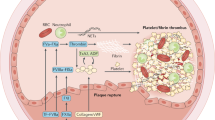
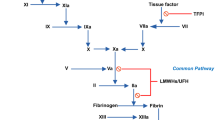
The generation of the proteolytic enzyme plasmin from its inactive precursor plasminogen, mediated by so called plasminogen activators, is the essential step in thrombolytic therapy. Plasmin is responsible for the degradation of the insoluble fibrin, the major component of a thrombus, to soluble fibrin degradation products. So far, the use of the more recently developed thrombolytic agents single-chain urokinase-type plasminogen activator (scu-PA) and tissue-type plasminogen activator (t-PA) were disappointing, mainly due to some of their negative propertiesin vivo, i.e., rapid inhibition and/or hepatic clearance. Besides some background information on the haemostatic balance; t-PA and scu-PA structure; and mechanisms of action, we here review some reported attempts to improve on these agents for thrombolytic therapy following various strategies. One of the more potential strategies, antibody-targeted thrombolytic therapy using bispecific monoclonal antibodies, is discussed somewhat more extensively, as are the several procedures that can befollowed for bispecific antibody preparation.
Similar content being viewed by others
Abbreviations
- bs-MoAb:
-
bispecific monoclonal antibody
- MoAb:
-
monoclonal antibody
- PAI-1:
-
plasminogen activator inhibitor type-1
- scu-PA:
-
single-chain urokinase-type plasminogen activator
- t-PA:
-
tissue-type plasminogen activator
- tcu-PA:
-
two-chain urokinase-type plasminogen activator
References
Doolittle RF. Fibrinogen and fibrin. Sci Am 1981; 245: 92–101.
Müllertz S. Fibrinolysis: an overview. Sem Thromb Haemostas 1984; 10: 1–5.
Wiman B, Collen D. On the kinetics of the reaction between antiplasmin and plasmin. Eur J Biochem 1987; 84: 573–8.
Collen D, Lijnen HR, Todd PA, Goa KL. Tissue-type plasminogen activator. A review of its pharmacology and therapeutic use as a thrombolytic agent. Drugs 1989; 38: 346–88.
Verstraete M, Bory M, Collen D, Erbel R, Lennane RJ, Mathey D, Michels HR, Schartl M, Uebis R, Bernard R, Brower RW, De Bono DP, Huhmann W, Lubsen J, Meyer J, Rutsch W, Von Essen R. Randomised trial of intravenous recombinant tissue-type plasminogen activator versus intravenous streptokinase in acute myocardial infarction. Lancet 1985; 1: 842–7.
The International Study Group. In-hospital mortality and clinical course of 20.891 patients with suspected acute myocardial infarction randomised between alteplase and streptokinase with or without heparin. Lancet 1990; 336: 71–5.
Brommer EJP. The level of extrinsic plasminogen activator (t-PA) during clotting as a determinant of the rate of fibrinolysis; inefficiency of activators added afterwards. Thromb Res 1984; 34: 109–15.
Kitaguchi H, Hijikata A, Hirata M. Effect of thrombin on plasminogen activator from isolated perfused dog heart. Thromb Haemostas 1985; 53: 126–32.
Rijken DC, Wijngaards G, Welbergen J. Relationship between tissue plasminogen activator and the activators in blood and vascular wall. Thromb Res 1979; 18: 815–30.
Levine EG, Loskutoff DJ. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol 1981; 94: 631–6.
Parekh RB, Dwek RA, Rudd PM, Thomas JR, Rademacher TW, Warren T, Wun T C, Herbert B, Reitz B, Palmier M, Ramahadran T, Tiemeier DC. N-glycosylation andin vitro enzymatic activity of human recombinant tissue plasminogen activator expressed in Chinese hamster ovary cells and a murine cell line. Biochemistry 1989; 28: 7670–9.
Parekh RB, Dwek RA, Thomas JR, Opdenakker G, Rademaker TW, Wittwer AJ, Howard SC, Nelson R, Siegel NR, Jennings MG, Harakas NK, Feder J. Celltype-specific and site-specific N-glycosylation of type I and type II human tissue plasminogen activator. Biochemistry 1989; 28: 7644–62.
13.Kluft C. t-PA in fibrin dissolution and haemostasis. In: Kluft C, ed. Tissue-type plasminogen activator (t-PA): physiological and clinical aspects. CRC Press, Boca Raton, 1988: 47–82.
Rijken DC. Structure /function relationships of t-PA. In: Kluft C, ed. Tissue-type plasminogen activator (t-PA): physiological and clinical aspects. CRC Press, Boca Raton, 1988: 101–22.
Degen SJF, Rajput B, Reich E. The human tissue plasminogen activator gene. J Biol Chem 1986; 261: 6972–85.
Van Zonneveld AJ, Veerman H, Pannekoek H. Autonomous functions of structural domains on humantissue-type plasminogen activator. Proc Natl Acad Sci USA 1986; 83: 4670–4.
Pannekoek H, De Vries C, Van Zonneveld AJ. Mutants of human tissue-type plasminogen activator (t-PA): structural aspects and functional properties. Fibrinolysis 1988; 2: 123–32.
Van Zonneveld AJ, Veerman H, Pannekoek H. On the interaction of the finger and the kringle-domain of tissuetype plasminogen activator with fibrin. Inhibition of kringle-2 binding to fibrin by e-amino caproic acid. J Biol Chem 1986; 261: 14214–8.
Verheijen JH, Caspers MPM, Chang GTG, De Munk GAW, Pouwels PH, Enger-Valk BE. Involvement of finger domain and kringle 2 domain of tissue-type plasminogen activator in fibrin binding and stimulation of activity by fibrin. EMBO J 1986; 5: 3525–30.
De Munk GAW, Caspers MPM, Chang GTG, Pouwels PH, Enger-Valk BE, Verheijen JH. Binding of tissuetype plasminogen activator to lysine, lysine analogues, and fibrin fragments. Biochem 1989; 28: 7318–25.
Verheijen JH, Caspers MPM, De Munk GAW, Enger-Valk BE, Chang GTG, Verwels PH. Sites in tissue-type plasminogen activator involved in the interaction with fibrin, plasminogen and low molecular weight ligands. Thromb Haemostas 1987; 58: 491–6.
Weening-Verhoeff EJD, Quax PHA, Van Leeuwen RTJ, Rehberg EF, Marotti KR, Verheijen JH. Involvement of aspartic and glutamic residues in kringle-2 of tissue-type plasminogen activator in lysin binding, fibrin binding and stimulation of activity as revealed by chemical modification and oligonucleotide-directed mutagenesis. Prot Eng 1990; 4: 191–8.
De Vries C, Veerman H, Pannekoek H. Identification of the domains of tissue-type plasminogen activator in the augmented binding to fibrin after limited digestion with plasmin. J Biol Chem 1989; 264: 12604–10.
Nieuwenhuizen W, Verheijen JH, Vermond A, Chang GTG. Plasminogen activation by tissue activator is accelerated in the presence of fibrin(ogen) cyanogen bromide fragment FCB2. Biochem Biophys Acta 1983; 755: 531–3.
Nieuwenhuizen W, Voskuilen M, Vermond A, Hoegeede Nobel B, Traas DW. The influence of fibrin(ogen) fragments on the kinetic parameters of the tissue-typeplasminogen-activator-mediated activation of different forms of plasminogen. Eur J Biochem 1988; 174: 163–9.
Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem 1982; 257: 2920–5.
Nieuwenhuizen W. Fibrinogen and its specific sites for modulation of t-PA induced fibrinolysis. In: Kluft C, ed. Tissue-type plasminogen activator (t-PA): physiological and clinical aspects. CRC Press, Boca Raton, 1988: 171–88.
Rijken DC, Hoylaerts M, Collen D. Fibrinolysic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem 1982; 257: 2920–5.
Kuiper J, Otter M, Rijken DC, Van Berkel ThJC. Characterization of the interactionin vivo of tissue-type plasminogen activator with liver cells. J Biol Chem 1988; 263; 18220–4.
Otter M, Kuiper J, Bos R, Rijken DC, Van Berkel ThJC. Characterization of the interaction bothin vitro andin vivo of tissue-type plasminogen activator (t-PA) with rat liver cells; effects of monoclonal antibodies to t-PA. In press.
Sobel BE, Sarnoff SJ, Nachowiak DA. Augmented and sustained plasma concentrations after intramuscular injections of molecular variants and deglycosylated forms of tissue-type plasminogen activators. Circulation 1990; 81: 1362–73.
Wu Z, Van De Werf F, Stassen T, Matson C, Pohl G, Collen D. Pharmacokinetics and coronary thrombolytic properties of two human tissue-type plasminogen activators variants lacking the finger-like, growth factor-like, and first kringle domains in a canine model. J Cardiovasc Pharmacol 1990; 16: 197–203.
Browne MJ, Carey JE, Chapman CG, Tyrell AWR, Entwisle C, Lawrence GMP, Esmail A, Robinson JH. A tissue-type plasminogen activator mutant with prolonged clearancein vivo. Effect of removal of the growth factor domain. J Biol Chem 1988; 263: 1599–1602.
Stump DC, Lijnen HR, Collen D. Purification and characterization of single-chain urokinase-type plasminogen activator from human cell cultures. J Biol Chem1986; 261: 1274–8.
De Munk GAW, Rijken DC. Fibrinolytic properties of single chain urokinase-type plasminogen activator (prourokinase). Fibrinolysis 1990; 4: 1–9.
Ichinose A, Fujikawa K, Suyama T. The activation of pro-urokinase by plasma kallikrein and its inactivation by thrombin. J Biol Chem 1986; 261: 3486–9.
Lijnen HR, Van Hoef B, Collen D. Activation with plasmin of two-chain urokinase-type plasminogen activator derived from single-chain urokinase-type plasminogen activator by treatment with thrombin. Eur J Biochem 1987; 169: 359–64.
Lijnen HR, Zamarron C, Blaber M, Winkler ME, Collen D. Activation of plasminogen by pro-urokinase.I. Mechanism. J Biol Chem 1986; 261: 1253–8.
Petersen LC, Lund LR, Nielsen LS, Dano K, Skriver L. One-chain urokinase-type plasminogen activator from human sarcoma cells is a proenzyme with little or no intrinsic activity. J Biol Chem 1988; 263: 11189–95.
Pannell R, Gurewich V. Pro-urokinase: a study of its stability in plasma and of a mechanism for its selective fibrinolytic effect. Blood 1986; 67: 1215–23.
Gurewich V. The sequential complementary and synergistic activation of fibrin-bound plasminogen by tissue plasminogen activator and pro-urokinase. Fibrinolysis 1989; 3: 59–66.
Declerck PJ, Lijnen HR, Verstreken M, Moreau H, Collen D. A monoclonal antibody specific for two chain urokinase-type plasminogen activator. Application to the study of the mechanism of clot lysis with single-chainurokinase-type plasminogen activator in plasma. Blood 1990; 75: 1794–1800.
Collen D, Mao J, Stassen JM, Broeze R, Lijnen HR. Thrombolytic properties of Lys-158 mutants of recombinant single chain urokinase-type plasminogen activator (scu-PA) in rabbits with jugular vein thrombosis. J Vasc Med Biol 1989; 1: 46–9.
Verheijen JH, Bakker AJ, Weening-Verhoeff EJD, Marotti KR, Rehberg E. Creation of a binding site for lysin and fibrin in kringle-1 of tissue-type plasminogen activator by substitution of six consecutive amino acids residues from the homologous kringle-2. Fibrinolysis 1990; 4: 165–72.
Kalyan NK, Wilhelm J, Lee SG, Dheer SK, Cheng S, Hjorth R, Pierzchala WA, Wiener F, Hung PP. Construction, expression and biochemical characterization ofa novel triskringle plasminogen activator gene. Fibrinolysis 1990; 4: 79–86.
Collen D, Lijnen HR, Bulens F, Vandamme AM, Tulinsky A, Nelles L. Biochemical and functional characterization of human tissue-type plasminogen activator var iants with mutagenized kringle domains. J Biol Chem 1990; 265: 12184–91.
Kaylan NK, Guang Lee S, Wilhelm J, Fu KP, Hum WT, Rappaport R, Hartzell RW, Urbano C, Hung PP. Structure function analysis with tissue-type plasminogen activator; effect of deletion of NH,-terminal domains on its biochemical and biological properties. J Biol Chem 1988; 263: 3971–8.
Markland W, Pollock D, Livingston DJ. Tissue-type plasminogen activator variants with domain duplications and rearrangemants. Prot Eng 1989; 3: 111–6.
Pohl G, Sterky C, Attersand A, Nyberg E, Löwenadler B, Hansson L. Tissue plasminogen activator mutants lacking the growth factor domain and the first kringle domain. I; DNA constructions, expression in mammalian cells, protein structure, fibrin affinity and enzymatic properties. Fibrinolysis 1991; 5: 17–29.
Wikström K, Mattson C, Sterky C, Pohl G. Tissue plasminogen mutants lacking the growth factor domain and the first kringle domain. II; Enzymatic properties in plasma andin vivo thrombolytic activity and clearance rates in rabbits. Fibrinolysis 1991; 5: 31–41.
Lijnen HR, Nelles L, Van Hoef B, De Clerck F, Collen D. Biochemical and functional characterization of human tissue-type plasminogen activator variants obtained by deletion and/or duplication of structural/ functional domains. J Biol Chem 1990: 265: 5677–83.
Larsen GR, Metzger M, Blue Y, Horgan P. Pharmacokinetic and distribution analysis of variant forms of tissue-type plasminogen activator with prolonged clearance in rat. Blood 1989; 73: 1842–50.
Trill JJ, Fong KL, Shebuski RJ, McDevitt P, Rosa MD, Johanson K, Williams D, Boyle KE, Sellers TS, Reff ME. Expression and characterization of finger protease (FP); a mutant tissue-type plasminogen activator (t-PA) with improved pharmacokinetics. Fibrinolysis 1990; 4: 131–40.
Collen D, Lijnen HR, Vanlinthout I, Kieckens L, Nelles L, Stasses JM. Thrombolytic and pharmacokinetic properties of human tissue-type plasminogen activators variants, obtained by deletion and/or duplication of structural/functional domains, in a hamster pulmonary embolism model. Thromb Haemostas 1991; 65: 174–80.
Ahern TJ, Morris GE, Barone KM, Horgan PG, Timony GA, Angus LB, Henson KS, Stoudemire JB, Langer-Safer PR, Larsen GR. Site-directed mutagenesis in human tissue-plasminogen activator. J Biol Chem 1990; 265: 5540–5.
Loscalzo J. Molecular biologic modifications of plasminogen activators; an artful science. Circulation 1990; 82:1062–3.
Maksimenko AV, Torchilin VP. Water-soluble urokinase derivatives with increased affinity to the fibrin clot. Thromb Res 1985; 38: 289–95.
Runge MS, Bode C, Matsueda GR, Haber E. Antibody enhanced thrombolysis: targeting of tissue plasminogen activatorin vivo. Proc Natl Acad Sci USA 1987; 84: 7659–62.
Bode C, Runge MS, Schönermark S, Eberle T, Newell JB, Kubler W, Haber E. Conjugation to antifibrin Fab' enhances fibrinolytic potency of single-chain urokinase plasminogen activator. Circulation 1990; 81: 1974–80.
Collen D, Dewerchin M, Rapold H, Lijnen HR, Stassen JM. Thrombolytic and pharmacokinetic properties of a conjugate of recombinant single-chain urokinase-type plasminogen activator with a monoclonal antibody specific for cross-linked fibrin in a baboon venous model. Circulation 1990; 82: 1744–53.
Schnee JM, Runge MS, Matsueda GR, Hudson NW, Seidman JG, Haber E, Quertermouse T. Construction and expression of a recombinant antibody-targeted plasminogen activator. Proc Natl Acad Sci USA 1987; 84: 6904–8.
Runge MS, Huang P, Savard CE, Schnee JM, Love TW, Bode C, Matsueda GR, Haber E, Quertermouse T. A recombinant antibody with antifibrin antibody and single chain urokinase activities has increased fibrinolytic potency. Circulation 1989; 78: 509.
Bode C, Runge MS, Branscomb EE, Newell JB, Matsueda GR, Haber E. Antibody directed fibrinolysis. An antibody specific for both fibrin and tissue plasminogen activator. J Biol Chem 1989; 264: 944–8.
Charpie JR, Runge MS, Matsueda GR, Haber E. A bispecific antibody enhances the fibrinolytic potency of single-chain urokinase. Biochem 1990; 29: 6374–8.
Kurokawa T, Iwasa S, Kakinuma A. Enhanced fibrinolysis by a bispecific monoclonal antibody reactive to fibrin and tissue plasminogen activator. Biotechnology 1989; 7: 1163–76.
Branscomb EE, Runge MS, Savard CE, Adams KM, Matsueda GR, Haber E. Bispecific monoclonal antibodies produced by somatic cell fusion increase the potency of tissue plasminogen activator. Thromb Haemostas 1990; 64: 260–6.
Bos R, Siegel K, Otter M, Nieuwenhuizen W. Production and characterization of a set of monoclonal antibodies against tissue-type plasminogen activator (t-PA). Fibrinolysis, in press.
Bos R, Otter M, Nieuwenhuizen W. Enhanced binding of t-PA to fibrin using bispecific monoclonal antibodies. In: Crommelin DJA and Schellekens H, eds. From Clone to Clinic. Kluwer Scientific Publishers, Dordrecht, the Netherlands, 1990: 167–74.
Bos R, Koolwijk P, Nieuwenhuizen W. Increased fibrin specificity of t-PA and u-PA, using a novel assay method. Thromb Haemostas 1991; 65: 779 (abstract ≠ 6374).
Lansdorp PM, Aalberse RC, Bos R, Schutter WG, Van Bruggen EJF. Cyclic tetramolecular complexes of monoclonal antibodies: a new type of cross-linking reagent. Eur J Immunol 1986; 16: 679–83.
Glennie MJ, Brennand DM, Bryden F, McBride HM, Stirpe F, Worth AAT, Stevenson GT. Bispecific F(AB'γ)2antibody for the delivery of saporin in thetreatment of lymphoma. J Immunol 1988; 141: 3662–70.
Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science 1985; 229: 81–3.
Millstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983; 305: 537–40.
Suresh MR, Cuello AC, Millstein C. Advantages of bispecific hybridomas in one-step immunocytochemistry and immunoassays. Proc Natl Acad Sci USA 1986; 83: 7989–93.
Karawajew L, Behrsing O, Kaiser G, Micheel B. Production and ELISA application of bispecific monoclonal antibodies against fluorescein isothiocyanate (FITC) and horseradish peroxidase (HRP). J Immunol Meth 1988; 111: 95–9.
Koolwijk P, Rozemuller E, Stad RK, De Lau WBM, Bast BJEG. Enrichment and selection of hybrid hybridomas by Percoll density gradient centrifugation and fluorescent-activated cell sorting. Hybridoma 1988; 7: 217–25.
Wright WE. The isolation of heterokaryons and hybrids by a selective system using irreversible biochemical inhibitors. Exp Cell Res 1987; 112: 395–407.
Stopper H, Zimmerman U, Neil GA. Increased efficiency of transfection of murine hybridoma cells with DNA by electroporation. J Immunol Meth 1988; 109: 145–51.
De Lau WBM, Van Loon AE, Heije K, Valerio D, Bast BJEG. Production of hybrid hybridomas based on HATS-NEOr double mutants. J Immunol Meth 1989; 117: 1–8.
Bos R, Nieuwenhuizen W. Enhanced transfection of a bacterial plasmid into hybridoma cells by electroporation: application for the selection of hybrid hybridoma (quadroma) cell lines. Hybridoma 1992; 11: 41–51.
Davies J, Smith DI. Plasmid determined resistance to antimicrobial agents. Ann Rev Microbiol 1978; 32: 469–518.
Mulligan RC, Berg P. Selection for animal cells that express the Escherichia coli gen coding for xanthineguanine phosphoribosyl transferase. Proc Natl Acad Sci USA 1981; 78: 2072–6.
Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promotor. J Mol Appl Genet 1982; 1: 327–32.
Bernard HU, Krämmer G, Röwekamp WG. Construction of a fusion gene that confers resistance against hygromycin-B onto mammalian cells in culture. Exp Cell Res 1985; 158: 237–43.
Tsong TY. On electroporation of cell membranes and some related phenomena. J Electroanal Chem 1990; 299: 271–95.
De Lau WBM, Heije K, Neefjes JJ, Oosterwegel M, Rozemuller E, Bast BJEG. Absence of preferential homologous heavy/light chain association in hybrid hybridomas. J Immunol 1991; 147: 906–9.
Koolwijk P, Spierenburg GT, Frasa H, Boot JHA, Van De Winkel JGJ, BastBJEG. Interaction between hybrid mouse monoclonal antibodies and the human High Affinity IgG FcR, HuFcγRI, on U937: involvement of only one of the mIgG heavy chains in receptor binding. J Immunol 1989; 143: 1556–662.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bos, R., Nieuwenhuizen, W. The potential improvement of thrombolytic therapy by targeting with bispecific monoclonal antibodies: Why they are used and how they are made. Biotherapy 5, 187–199 (1992). https://doi.org/10.1007/BF02171051
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02171051