Abstract
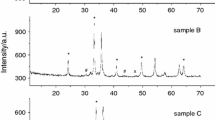
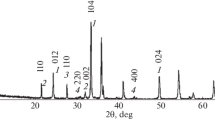
Thermal decomposition of lithium tris (malonato) ferrate (III) tetrahydrate i.e. Li3[Fe(CH2C2O4)3].4H2O has been studied in the temperature range of 353–873 K in static air atmosphere using Mössbauer, infrared spectroscopy and nonisothermal techniques (TG-DTG-DTA). The anhydrous complex decomposed into ferric oxide of varying particle sizes and alkali metal malonates/carbonates in succesive stages. Fimally a solid state reaction between α-Fe2O3 and alkali metal carbonate gives fine particles of lithium ferrite (LiFeO2) at a temperature lower than for oxalate precursor and for ceramic method.
Similar content being viewed by others
References
A. Srivastva, P. Singh, V.G. Gunjikar, A.P.B. Sinha,Thermochim. Acta, 86 (1985) 77.
B.S. Randhawa, Sandeep Kaur, P.S. Bassi,Indian J. Chem., 28A (1989) 463.
P.S. Bassi, B.S. Randhawa, Sandeep Kaur, Proc. 5th Int. Conf. on Ferrites, 1989, p. 67.
P.S. Bassi, B.S. Randhawa, Sandeep Kaur,Indian J. Chem., 31A (1992) 596.
Ferrite Materials, (Eds by B. Viswanathan, V.R.K. Murthy) Narosa Publ. House, New Delhi, 1990, p. 82.
P.S. Bassi, B.S. Randhawa, Sandeep Kaur,Hyperfine Interactions, 28 (1986) 745.
J.R. Ferraro, R. Driver, W.R. Walker, W. Wozniak,Inorg. Chem., 6 (1967) 1586.
M. Blume,Phys. Rev. Lett., 14 (1965) 96.
A. Vértes, L. Korecz, K. Burger, Mössbauer Spectroscopy, Elsevier, New York, 1979, p. 47.
D. Barb, L. Diamandescu, D. Mihaila-Tarabasanu,Chem. Phys., 44 (1979) 239.
R.A. Nyquist, R.O. Kagel, Infrared Spectra of Inorganic Compounds, Academic Press, New York, 1971.
W. Kundig, H. Bommel, G. Constabaris, R.H. Lindquist,Phys. Rev., 142 (1966) 327.
O. Knop, C. Ayarsa, J.S. Carlow, W.W. Baker,J. Solid State Chem., 25 (1978) 329.
T. Ichida, T. Shinjo, Y. Bando, T. Takada,J. Phys. Soc. Japan, 29 (1970) 795.
T. Ichida, T. Shinjo, Y. Bando, T. Takada,J. Phys. Soc. Japan, 29 (1970) 1109.
A.S. Brar, B.S. Randhawa,J. Solid State Chem., 58 (1985) 153.
K. Nagase, K. Muraishi, K. Sone, N. Tanaka,Bull. Chem. Soc. Japan, 48 (1975) 3184.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Randhawa, B.S., Bassi, P.S. & Kaur, S. Thermal analysis of lithium tris (malonato) ferrate(III) tetrahydrate. Journal of Radioanalytical and Nuclear Chemistry Letters 188, 279–286 (1994). https://doi.org/10.1007/BF02164888
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02164888