Abstract
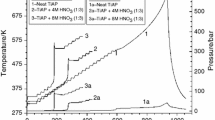
The thermal decomposition of γ-irradiated magnesium bromate was studied by dynamic thermogravimetry. The reaction order, activation energy, frequency factor and entropy of activation were calculated by the Coats-Redfern equation and were compared with those of the unirradiated salt. Irradiation enhances the decomposition and the effect increases with irradiation dose. The activation energy decreases on irradiation. The mechanism for the decomposition of unirradiated and irradiated magnesium bromate follows the Avrami model equation, 1-/1-∞/1/3=kt, and the rate-controlling, process is a phase boundary reaction assuming spherical symmetry.
Similar content being viewed by others
References
S.M.K. Nair, C. James,Thermochim. Acta, 96 /1985/ 27.
S.M.K. Nair, K.K. Malayil, P.D. Jacob,Thermochim. Acta /in press/.
S.M.K. Nair, P.D. Jacob,Thermochim. Acta /communicated/.
S.R. Mohanty,J. Sci. Res., Banaras Hindu University, 12 /1961/ 299.
S.R. Mohanty, V.M. Pandey,J. Sci. Ind. Res., 34 /1975/ 196.
J.W. Chase, G.E. Boyd,J. Phys. Chem., 70 /1966/ 1031.
J. Sestak, G. Berggren,Thermochim. Acta, 3 /1971/ 1.
V. Satava,Thermochim. Acta, 2 /1971/ 423.
W.W. Wendlandt, Thermal Methods of Analysis, J. Wiley and Sons, 1974, p. 45.
G.M. Bancroft, H.D. Gesser,J. Inorg. Nucl. Chem., 27 /1965/ 1545.
H.T.S. Britton, H.G. Britton,J. Chem. Soc., /1952/ 3887.
G.E. Boyd, E.W. Graham, Q.V. Larson,J. Phys. Chem., 66 /1962/ 300.
A.W. Coats, J.P. Redfern,Nature /London/, 201 /1964/ 68.
E.S. Freeman, B. Carroll,J. Phys. Chem., 62 /1958/ 394.
H.H. Horowitz, G. Metzger,Anal. Chem., 35 /1963/ 1464.
S.R. Dharwadkar, M.D. Karkhanavala /Eds. R.F. Schwenker, Jr., P.D. Garns/, in: Thermal Analysis, Vol. 2. Proc. 2nd ICTA, Worcester, MA, 1968. Academic Press, New York 1969, pp. 1049–1069.
N.A. Lange, G.M. Forker /Eds./, Handbook of Chemistry, 10th ed., McGraw-Hill, New York, 1961, pp. 266–267.
J. Jach,J. Phys. Chem. Solids, 24 /1963/ 63.
E.J. Prout,J. Inorg. Nucl. Chem., 7 /1958/ 368.
S.D. Bhattmaisra, S.R. Mohanty,Rad. Eff., 29 /1976/ 41.
S.M.K. Nair, C. James,Thermochim. Acta, 78 /1984/ 357.
S.M.K. Nair, C. James,Thermochim. Acta, 83 /1985/ 387.
J. Sestak,Thermochim. Acta, 3 /1971/ 150.
M. Avrami,J. Chem. Phys., 7 /1939/ 103.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nair, S.M.K., Jacob, P.D. The effect of γ-irradiation on the thermal decomposition of magnesium bromate. Journal of Radioanalytical and Nuclear Chemistry Letters 137, 113–125 (1989). https://doi.org/10.1007/BF02164574
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02164574