Abstract
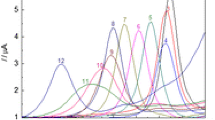
Differential pulse voltammetric methods have been developed for the simultaneous estimation of the constituents of uranium-iron and uranium-cadmium mixtures in solution. A mixture of 1M H3PO4−1M KH2PO4 (with a pH≈1.5), was found to be the most ideal supporting electrolyte for both methods, among many that were evaluated for their suitability. In uranium-iron mixtures the calibration for iron was found to be linear up to 150 μg ml−1 (r2=0.9986), while that of uranium up to 500 μg ml−1 (r2=0.999). Iron at 6.7 μg ml−1 level could be determined in the presence of 800 fold uranium (wt/wt) without significant interference. Uranium at 21 μg ml−1 level could be analyzed with 5-fold iron (wt/wt). This upper limit of iron was due to the precipitation of iron as phosphate. In the case of uranium — cadmium mixtures, cadmium calibration for cadmium was found to be linear up to 1300 μg ml−1 (r2=0.9993). Concentration levels of 4.6 μg ml−1 Cd could be determined at a 500-fold excess (wt/wt) of uranium. Uranium calibration was linear up to 500 μg ml−1 (r2=0.999) and 21 μg ml−1 uranium could tolerate up to a 1000-fold excess of cadmium (wt/wt). Both procedures could tolerate 10 μg ml−1 levels of metal ions, such as chromium, copper, manganese, molybdenum and vanadium.
Similar content being viewed by others
References
N. Srinivasan, M.N. Nadkarni, G.R. Balasubramanian, R.T. Chitnis, H.R. Siddique, Report BARC-643 (1972).
B.F. Scribner, H.R. Mullin, Journal of Research National Bureau of Standards, 37, 379–389, Dec. 1946.
A.R. Joshi, U.M. Kasar,J. Radioanal. Nucl. Chem., Lett., 146 (1990) 401.
T. Fujinaga, H.L. Lee,Talanta, 24(6) (1977) 395.
I.M. Kolthoff, P.J. Elving, Treatise on Analytical Chemistry, Interscience Publication, Part-II, Vol. 9., p. 74.
Y.I. Chang, Report No. ANL/CP-75576 (1992).
Principles and Practice of Analytical Chemistry, (Eds by F.W. Fifield and D. Kealy), p. 216.
W.E. Harris, I.M. Kolthoff,J. Am. Chem. Soc., 67 (1945) 1484.
H.J. Shalgosky, AERE, Harwell, Report C/R 1869 (1956).
W.E. Harris, I.M. Kolthoff,J. Am. Chem. Soc., 68 (1946) 1178.
D. T. Sawyer, J.U. Roberts, Jr., Experimental Electrochemistry for Chemists, Wiley Interscience Publication, p. 365.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sreenivasan, N.L., Srinivasan, T.G. Differential pulse voltammetric methods for the simultaneous estimation of uranium-iron and uranium-cadmium mixtures in solution. Journal of Radioanalytical and Nuclear Chemistry Letters 201, 391–399 (1995). https://doi.org/10.1007/BF02164215
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02164215