Summary
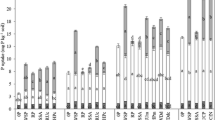
The P-supplying power of triple superphosphate, three apatitic rock phospates and a calcined aluminum rock phosphate were tested by measuring the quantities of fertilizer P recovered in soybean and in four chemical extractants, after 3-day and 75-day periods of contact between soil and fertilizer.
The triple superphosphate supplied the highest amounts of P, but it lost efficiency during the longer incubation period. The rock phosphates maintained their original efficiencies, probably as a result of a balance between P released from the fertilizer and P converted into non-labile forms.
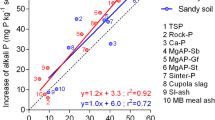
The following coefficients of correlation between P uptake by soybean from an acid oxisol and P extracted by chemical extractants, after the two incubation periods, were found: 0.902** for 0.01M CaCl2; 0.823** for anion-exchange resin; 0.720** for 0.03N NH4F+0.025N HCl; −0.037 (n.s.) for 0.025N H2SO4+0.050N HCl.
The acid NH4F solubilized residual calcined aluminum phosphate particles, and double acid extracted P from residual apatite particles, thus accounting for their poorer performances in predicting availability of fertilizer P.
The relative efficiencies of the rock phosphates could largely be predicted after an incubation period of only three days. This finding attests to the presence in these rock phosphates of an easily soluble fraction of P which is not indicative of the degree of reactiveness of the phosphate as a whole.
Similar content being viewed by others
References
Arndt, W. and McIntyre, G. A. 1963 The initial and residual effects of superphosphate and rock phosphate for sorghum on a lateritic red earth. Aust. J. Agric. Res.14, 785–795.
Barnes, J. S. and Kamprath, E. J. 1975 Availability of North Carolina rock phosphate applied to soils. Tech. Bull., North Carolina Agric. Exp. Stn. No.229, 21 p.
Barrow, N. J. 1974 The slow reactions between soil and anions: 1. Effects of time, temperature and water content of a soil on the decrease in effectiveness of phosphate for plant growth. Soil. Sci.118, 380–386.
Chien, S. H. 1977 Dissolution rates of phosphate rocks. Soil Sci. Soc. Am. J.41, 656–657.
Devine, J. R., Gunary, D. and Larsen, S. 1968 Availability of phosphate as affected by duration of fertilizer contact with soil. J. Agric. Sci.71, 359–369.
Ellis, R. M., Quader, M. A. and Truog, E. 1955 Rock phosphate availability as influenced by soil pH. Soil Sci. Soc. Am Proc.19, 484–487.
Ensminger, L. E., Pearson, R. W. and Armiger, W. H. 1967 Effectiveness of rock phosphate as a source of phosphorus for plants. U.S.D.A., A.R.S., 41–125.
Fox, R. L. and Kamprath, E. J. 1960 Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Sci. Soc. Am. Proc.34, 902–906.
Hignett, T. P., Doll, E. C., Livingston, O. W. and Raistrick, B. 1977 Utilization of difficult phosphate ores.In New Developments in Phosphate Fertilizer Technology. Ed. L. J. Carpentier. Elsevier Publishing Comp., Amsterdam, 274–288.
Jackson, J. E. and Burton, G. W. 1960 A five-year comparison of rock phosphate and concentrated superphosphate for several forage crops. Agron. J.52, 692–694.
Khasawneh, F. E. and Doll, E. C. 1978 The use of phosphate rock for direct application to soils. Adv. Agron.30, 159–206.
Larsen, S. and Probert, M. E. 1968 A theoretical treatment of the maintenance of soil phosphorus status. Phosphorus in Agric.51, 1–6.
Lehr, J. R. and McClellan, G. H. 1972 A revised laboratory reactivity scale for evaluating phosphate rocks for direct application. Bulletin Y-43, Tennessee Valley Authority, Muscle Shoals, Alabama.
Marten, D. C., Lutz, J. A. and Jones, G. D. 1969 Form and availability of phosphorus in selected Virginia soils as related to available phosphorus tests. Agron. J.61, 616–621.
Minhas, R. S. and Kick, H. 1974 Comparative availability of superphosphate and rock phosphate and their distribution into different inorganic phosphate fractions after addition of heavy doses. Fert. News19, 12–16.
Paauw, F. van der 1965 Factors controlling the efficiency of rock phosphates for potatoes and rye on humic sandy soils. Plant and Soil22, 82–98.
Smith, A. N. 1968 The uptake of phosphorus by wheat and clover from four inorganic soil phosphate fractions after the addition of contrasting types of phosphatic fertilizers. Plant and Soil29, 144–155.
Thomas, G. W. and Peaslee, D. E. 1973 Testing soils for phosphorus.In Soil Testing and Plant Analysis, revised ed. Ed. L. M. Walsh and J. D. Beaton. Soil Sci. Soc. Am. Inc., Madison, U.S.A., 115–132.
Wright, D. N. 1975 Calcined Christmas Island C-grade rock phosphate as a fertilizer. Aust. J. Exp. Agric. Animal Husb.15, 419–423.
Author information
Authors and Affiliations
Additional information
on leave at the Agricultural University during 1977.
Rights and permissions
About this article
Cite this article
Van Raij, B., Van Diest, A. Phosphate supplying power of rock phosphates in an oxisol. Plant Soil 55, 97–104 (1980). https://doi.org/10.1007/BF02149713
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02149713