Summary
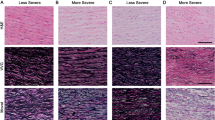
The aortic walls of patients with abdominal aortic aneurysms (AAA) and of healthy controls were examined for elastin, collagen I and III, and the intermediate filament proteins desmin and vimentin by immunohistochemical, enzyme histochemical, and routine histological techniques. The morphology of the aneurysmatic walls varied considerably from case to case, but many pathological changes were seen in all cases, e.g., extensive atherosclerotic plaques in the intima, prominent alterations in amount and organization of the elastic lamellae in the media, and an increase of connective tissue. Both collagen I and III were present in all the aneurysmatic walls. The smooth muscle cells in all the aortic walls showed a marked heterogeneity with respect to the morphological appearance, the enzyme histochemical features, and the content of desmin and vimentin. Vimentin occurred in some intimal, medial muscle, and adventitial cells of both the controls and the AAA patients. Desmin occurred in some of the intimal, medial, and adventitial muscle cells of both the controls and the AAA patients. All the cells with desmin in the intima and media also contained vimentin. Thus, smooth muscle cells in the walls of both the normal human abdominal aorta and aneurysms contained either vimentin, desmin, or both. This variability may be explained by the presence of different phenotypes of smooth muscle cells and could be of significance for the development of atherosclerosis and aneurysms. Of special interest was the finding that 5 of the 24 AAA patients studied had blood relatives with the same disease, suggesting a hereditary influence. However, no systematic differences between the morphological appearance of the aneurysmatic walls in familial and nonfamilial AAA could be detected.
Similar content being viewed by others
References
Zarins CK, Glagov S (1982) Aneurysms and obstructive plaques: Differing local response to atherosclerosis. In: Bergan JJ, Yao JST (eds) Aneurysms, diagnosis and treatment. Grune and Stratton, New York, pp 61–82
Constantinides P (1983) Ultrastructural pathobiology. Elsevier, Amsterdam, pp 99–138
Haust D (1983) Atherosclerosis-lesions and sequelae. In: Silver MD (ed) Cardiovascular pathology, vol 1. Churchill Livingstone, New York, pp 191–315
Norrgård Ö, Rais O, Ängquist K-A (1984) Familial occurrence of abdominal aortic aneurysms. Surgery 95: 650–656
Norrgård Ö (1985) Familial occurrence of abdominal aortic aneurysms (thesis). New Series no. 133. ISSN 0346-6612. Umeå University, Umeå
Wissler RW (1978) Progression and regression of atherosclerotic lesions. In: Chandler AB, Mac Millan GC, Nelson CB, Schwartz CJ, Wessler S (eds) Advances in experimental medicine and biology, vol 104. Plenum, New York, pp 77–109
Berenson GS, Radhakrishnamurthy B, Srinivasan SR, Vijayagopal P, Dalferes ER, Sharma C (1984) Recent advance in molecular pathology. Exp Mol Pathol 41: 267–287
McGill HC Jr (1984) Persistent problems in the pathogenesis of atherosclerosis. Arteriosclerosis 4: 443–451
Campbell GR, Chamley-Campbell JH (1981) Invited review. The cellular pathobiology of atherosclerosis. Pathology 13: 423–440
Campbell GR, Campbell JH (1985) Recent advances in molecular pathology, Smooth muscle phenotypic changes in arterial wall homeostasis: Implications for the pathogenesis of atherosclerosis. Exp Mol Pathol 42: 139–162
Berner PF, Frank E, Holtzer H, Somlyo AP (1981) The intermediate filament protein of rabbit vascular smooth muscle: Immunofluorescent studies of desmin and vimentin. J Muscle Res Cell Motil 2: 439–452
Frank ED, Warren L (1981) Aortic smooth muscle cells contain vimentin instead of desmin. Proc Natl Acad Sci USA 78: 3020–3024
Osborn M, Caselitz J, Weber K (1981) Heterogeneity of intermediate filament expression in vascular smooth muscle: A gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation 20: 196–202
Schmid E, Osborn M, Rungger-Brändle E, Gabbiani G, Weber K, Franke WW (1982) Distribution of vimentin and desmin filaments in smooth muscle tissue of mammalian and avian aorta. Exp Cell Res 137: 329–340
Gabbiani G, Rungger-Brändle E, de Chastonay C, Franke WW (1982) Vimentin-containing smooth muscle cells in aortic intimal thickening after endothelial injury. Lab Invest 47: 265–269
Padykula HA, Herman E (1955) The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem 3: 170–183
Brooke MH, Kaiser KK (1970) Muscle fiber types: How many and what kind? Arch Neurol 23: 369–379
Engel WK, Cunningham CG (1963) Rapid examination of muscle tissue. An improved trichrome method for fresh-frozen biopsy sections. Neurology 13: 919–923
Dubowitz V, Brooke MH (1973) Muscle biopsy: A modern approach. Saunders, London
Stephens HR, Duance VC, Dunn MJ, Bailey AJ, Dubowitz V (1982) Collagen types in neuromuscular diseases. J Neurol Sci 53: 54–62
Eriksson A, Thornell L-E, Stigbrand T (1978) Cytoskeletal filaments of heart conducting system localized by antibody against a 55,000 dalton protein. Experientia 34: 792–794
Gay S, Miller EJ (1983) What is collagen, what is not. Ultrastruct Pathol 4: 365–377
McCullagh KA, Balian G (1975) Collagen characterisation and cell transformation in human atherosclerosis. Nature 258: 73–75
Ooshima A (1981) Collagen B chain: Increased proportion in human atherosclerosis. Science 213: 666–668
McCullagh KG, Duance VC, Bishop KA (1980) The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol 130: 45–55
Gay S, Miller EJ (1978) Collagen in the physiology and pathology of connective tissue. Fisher, New York
Glagov S (1972) Hemodynamic risk factors: Mechanical stress, mural architecture, medial nutrition, and the vulnerability of arteries to atherosclerosis. In: Wissler RW, Geer JC (eds) The pathogenesis of atherosclerosis. William and Wilkins, Baltimore
Yoshida Y, Suzuki K, Shinkai H, Ooneda G (1980) Morphological studies on age changes of arterial walls and progression of atherosclerosis in human aorta and cerebral arteries, and effects of lipoproteins on proliferation of monkey aortic smooth muscle cells. Jpn Circ J 44: 13–32
Dingemans KP, Jansen N, Becker AE (1981) Ultrastructure of the normal human aortic media. Virchows Arch A (Pathol Anat) 392: 199–216
Leushner JRA, Haust MD (1986) Interstitial collagens in filbrous atherosclerotic lesions of human aorta. Path Biol 34: 14–18
Pope FM, Narcisi P, Neil-Dwyer G, Nicholls AC, Bartlett J, Doshi B (1981) Some patients with cerebral aneurysms are deficient in type III collagen. Lancet II: 973–975
Steinert PM, Jones JCR, Goldman RD (1984) Intermediate filaments. J Cell Biol 99: 22s-27s
Osborn M, Altsmannsberger O, Debus E, Weber K (1984) Conventional and monoclonal antibodies to intermediate filament proteins in human tumor diagnosis. Cancer Cells 1: 191–200
Gabbiani G, Schmid E, Winter S, Chaponnier C, de Chastonay C, Vandekerckhove J, Weber K, Franke WW (1981) Vascular smooth muscle cells differ from other smooth muscle cells: Predominance of vimentin filaments and a specific α-type actin. Proc Natl Acad Sci USA 78: 298–302
Campbell GR, Chamley-Campbell JH, Burnstock G (1981) Differentiation and phenotypic modulation of arterial smooth muscle cells. In: Schwartz CJ, Werthessen NT, Wolf S (eds) Structure and function of the circulation, vol 3. Plenum, New York pp 357–399
Ross R, Wight TN, Strandness E, Thiele B (1984) Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thornell, LE., Norrgård, Ö., Eriksson, A. et al. Abdominal aortic aneurysms: Distribution of elastin, collagen I and III, and intermediate filament proteins desmin and vimentin—A comparison of familial and nonfamilial aneurysms. Heart Vessels 2, 172–183 (1986). https://doi.org/10.1007/BF02128144
Issue Date:
DOI: https://doi.org/10.1007/BF02128144