Abstract
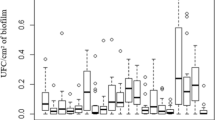
The influence of various incubation atmospheres on the growth and slime production of 23Staphylococcus epidermidis strains was studied. The atmospheres evaluated were aerobiosis (control), anaerobiosis, candle jar, 5 % CO2 and 10 % CO2. As compared to the aerobic control, growth was 55.7 ± 19 % (p<0.01) in anaerobic incubation, 113.7 ± 12 % (p<0.01) in 5 % CO2, 112.8 ± 13 % (p<0.01) in 10 % CO2 and 106.4 ± 7 % (p>0.1) in the candle jar. The slime production in relation to the aerobic control was 20.3 ± 19 % in anaerobiosis (p<0.01), 22.3 ± 27 % (p<0.01) in 5 % CO2, 29.4 ± 39 % (p<0.01) in 10 % CO2 and 68.3 ± 26 % (p>0.1) in the candle jar. The results of this study may explain the discrepancies which have been noted on occasion between slime formation data and pathogenicity.
Similar content being viewed by others
References
Davenport DS, Massanari RM, Pfaller MA, Bale MJ, Streed SA, Hierholzer WJ: Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. Journal of Infectious Diseases 1986, 153: 332–339.
Deighton MA, Balkau B: Adherence measured by microtiter assay as a virulence marker forStaphylococcus epidermidis infections. Journal of Clinical Microbiology 1990, 28: 2442–2447.
Younger JJ, Christensen GD, Bartley DL, Simmons CH, Barrett FF: Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. Journal of Infectious Diseases 1987, 156: 548–554.
Anwar H, Strap JL, Costerton JW: Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrobial Agents and Chemotherapy 1992, 36: 1347–1351.
Johnson GM, Lee DA, Regelmann WE, Gray ED, Peters G, Quie PG: Interference with granulocyte function byStaphylococcus epidermidis slime. Infection and Immunity 1986, 54: 13–20.
Peters G: New considerations in the pathogenesis of coagulase-negative staphylococcal foreign body infections. Journal of Antimicrobial Chemotherapy 1988, 21, Supplement C: 139–148.
Alexander W, Rimland D: Lack of correlation of slime production with pathogenicity in continuous ambulatory peritoneal dialysis peritonitis caused by coagulase negative staphylococci. Diagnostic Microbiology and Infectious Diseases 1987, 8: 215–220.
Kristinsson KG, Spencer RC: Slime production as a marker for clinically significant infections with coagulase negative staphylococci. Journal of Infectious Diseases 1986, 154: 728.
Barker LP, Simpson WA, Christensen GD: Differential production of slime under aerobic and anaerobic conditions. Journal of Clinical Microbiology 1990, 28: 2578–2579.
Ganong WF: Review of medical physiology. Lange Medical Publications, Los Altos, California, 1983, p. 533–539.
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH: Adherence of coagulase negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. Journal of Clinical Microbiology 1985, 22: 996–1006.
Martín-Andres A, Luna del Castillo JD: Bioestadística para las ciencias de la salud. Norma, Madrid, 1994, p. 401–430.
Culebras JM: Transporte de gases en la sangre. In: Herrera H (ed): Bioquímica. Volume II. Interamericana, Barcelona, 1991, p. 1345–1360.
Denyer SP, Davies MC, Evans JA, Finch RG, Smith DGE, Wilcox MH, Williams P: Influence of carbon dioxide on the surface characteristics and adherence potential of coagulase-negative staphylococci. Journal of Clinical Microbiology 1990, 28: 1813–1817.
Wilcox MH, Finch RG, Smith DGE, Williams P, Denyer P: Effects of carbon dioxide and sub-lethal levels of antibiotics on adherence of coagulase-negative staphylococci to polystyrene and silicone rubber. Journal of Antimicrobial Chemotherapy 1991, 27: 577–587.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pérez-Giraldo, C., Rodríguez-Benito, A., Morán, F.J. et al. Influence of the incubation atmosphere on the production of slime byStaphylococcus epidermidis . Eur. J. Clin. Microbiol. Infect. Dis. 14, 359–362 (1995). https://doi.org/10.1007/BF02116534
Issue Date:
DOI: https://doi.org/10.1007/BF02116534