Abstract
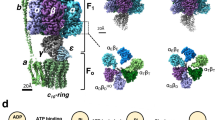
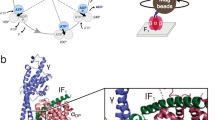
Exchange-out of amide tritium from labeled γ-subunit of α3β3γ complex of F0F1-ATP synthase was not accelerated by ATP, suggesting that hemagglutinin-type transition of coiled-coil structure did not occur in γ-subunit. Local topology of nucleotide binding site and “switch II” region of G-protein α resemble those of F1-β subunit and other proteins which catalyze ATP-triggered reactions. Probably, binding of nucleotide to F0F1-ATP synthase induces conformational change of the switch II-like region with transforming β subunit structure from “open” to “closed” form and this transformation results in loss of hydrogen bonds with the γ subunit, thus enabling the γ subunit to move.
Similar content being viewed by others
References
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994).Nature 370, 621–628.
Amano, T., Tozawa, K., Yohsida, M., and Murakami, H. (1994a).FEBS Lett. 348, 93–98.
Amano, T., Yoshida, M., Matsuo, Y., and Nishikawa, K. (1994b)FEBS Lett. 351, 1–5.
Boyer, P. D. (1993).Biochim. Biophys. Acta 1140, 215–250.
Bullough, P. A., Hughson, F. M., Skehel, J. J., and Wiley, D. C. (1994).Nature 371, 37–43.
Duncan, T. M., Bulygin, V. V., Zhou, Y., Hutcheon, M. L., and Cross, R. L. (1995).Proc. Natl. Acad. Sci. USA 92, 10964–10968.
Economou, A., Pogliano, J. A., Beckwith, J., Oliver, D. B., and Wickner, W. (1996).Cell 883, 1171–1182.
Ferguson, S. J., Lloyd, W. J., Lyons, M. H., and Radda, G. K. (1975).Eur. J. Biochem. 54, 117–126.
Foster, D. L., and Fillingame, R. H. (1982).J. Biol. Chem. 257, 2009–2015.
Georgiadis, M. M., Komiya, H., Chakrabarti, P., Woo, D., Kornuc, J. J., and Rees, D. C. (1992).Science 257, 1653–1659.
Hermolin, J., and Fillingame, R. H. (1989).J. Biol. Chem. 264, 3896–3903.
Kaibara, C., Matsui, T., Hisabori, T., and Yoshida, M. (1996).J. Biol. Chem. 271, 2433–2438.
Kimura, E., Akita, M., Matsuyama, S., and Mizushima, S. (1991).J. Biol. Chem. 266, 6600–6606.
Kurumizawa, H., and Shibata, T. (1996).J. Biochem. (Tokyo) 119, 216–223.
Lambright, D. G., Noel, J. P., Hamm, H. E., and Sigler, P. B. (1994).Nature 369, 621–628.
Lambright, D. G., Sondek, J., Bohm, A., Skiba, N. P., Hamm, H. E., and Sigler, P. B. (1996).Nature 379, 311–319.
Mitchell, P. (1966).Biol. Rev. 41, 455–502.
Mittal, R., Erickson, J. W., and Cerione, R. A. (1996).Science 271, 1413–1416.
Mixon, M. B., Lee, E., Coleman, D. E., Berghuis, A. M., Gilman, A. G., and Sprang, S. R. (1995).Science 270, 954–960.
Normand, P., and Bousquet, J. (1989).J. Mol. Evol. 29, 436.
Ohta, S., Tsuboi, M., Yoshida, M., and Kagawa, Y. (1980).Biochemistry 19, 2160–2165.
Ohtsubo, M., Yoshida, M., Ohta, S., Kagawa, Y., Yohda, M., and Date, T. (1987).Biochem. Biophys. Res. Commun. 146, 705–710.
Pedersen, P. L., and Amzel, L. M. (1993).J. Biol. Chem. 268, 9937–9940.
Ryrie, I. J., and Jagendorf, D. T. (1972).J. Biol. Chem. 247, 4453–4459.
Senior, A. E. (1990).Annu. Rev. Biophys. Chem. 19, 7–41.
Story, R. M., and Steiz, T. A. (1992).Nature 355, 374–376.
Story, R. M., Webber, I. T., and Steitzs, T. A. (1992).Nature 355, 318–325.
Walker, J. E., Saraste, M., Runswick, M. J., and Gay, N. J. (1982).EMBO J. 1, 945–951.
Wall, M. A., Coleman, D. E., Lee, I.-L., J. A., Posner, B. A., Gilman, A. G., and Sprang, S. R. (1995).Cell 83, 1047–1058.
Watts, S. D., Zahng, Y., Fillingame, R. H., and Capaldi, R. A. (1995).FEBS Lett. 368, 235–238.
Wiley, D. C., and Skehel, J. J. (1987).Annu. Rev. Biochem. 56, 365–394.
Yoshida, M., and Allison, W. S. (1983).J. Biol. Chem. 258, 14407–14412.
Yoshida, M., and Amano, T. (1994).FEBS Lett. 359, 1–5.
Yoshida, M., Sone, N., Hirata, H., Kagawa, Y., and Ui, N. (1979).J. Biol. Chem. 254, 9525–9533.
Yoshida, M., Poser, J. W., Allison, W. S., and Esch, F. S. (1981).J. Biol. Chem 256, 148–153.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Noji, H., Amano, T. & Yoshida, M. Molecular switch of F0F1-ATP synthase, G-protein, and other ATP-driven enzymes. J Bioenerg Biomembr 28, 451–457 (1996). https://doi.org/10.1007/BF02113988
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02113988