Abstract
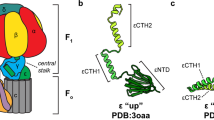
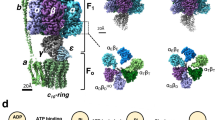
Structural changes in theEscherichia coli ATP synthase (ECF1F0) occur as part of catalysis, cooperativity and energy coupling within the complex. The γ and ε subunits, two major components of the stalk that links the F1 and F0 parts, are intimately involved in conformational coupling that links catalytic site events in the F1 part with proton pumping through the membrane embedded F0 sector. Movements of the γ subunit have been observed by electron microscopy, and by cross-linking and fluorescence studies in which reagents are bound to Cys residues introduced at selected sites by mutagenesis. Conformational changes and shifts of the ε subunit related to changes in nucleotide occupancy of catalytic sites have been followed by similar approaches.
Similar content being viewed by others
References
Abrahams, J. P., Leslie, A. G. W., Lutter, R., and Walker, J. E. (1994).Nature 370, 621–628.
Aggeler, R., and Capaldi, R. A. (1993).J. Biol. Chem. 268, 14576–145.
Aggeler, R., and Capaldi, R. A. (1996),J. Biol. Chem. 271, in press.
Aggeler, R., Chicas-Cruz, K., Cai, S.-X., Keana, J. F. W., and Capaldi,Biochemistry 31, 2956–2961.
Aggeler, R., Cai, S. X., Keana, J. F. W., Koike, T., and Capaldi, R. A. (1993).J. Biol. Chem. 268, 20831–20837.
Aggeler, R., Weinreich, F., and Capaldi, R. A. (1995a).Biochim. Biophys. Acta 1230, 62–68.
Aggeler, R., Haughton, M. A., and Capaldi, R. A. (1995b).J. Biol. Chem. 270, 9185–9191.
Boyer, P. D. (1993).Biochim. Biophys. Acta 1440, 215–250.
Capaldi, R. A. (1994).Nature Struct. Biol. 1, 660–663.
Capaldi, R. A., Aggeler, R., Turina, P., and Wilkens, S. (1994).Trends Biol. Sci. 19, 284–289.
Dallmann, H. G., Flynn, T. G., and Dunn, S. D. (1992).J. Biol. Chem. 267, 18953–18960.
Duncan, T. M., Bulygin, V. V., Zhou, Y., Hutcheon, M. L., and Cross, R. L. (1995).Proc. Natl. Acad. Sci. USA 92, 10964–10968.
Gogol, E. P., Johnston, E., Aggeler, R., and Capaldi, R. A. (1990).Proc. Natl. Acad. Sci. USA 87, 9585–9589.
Kuki, M., Noumi, T., Maeda, M., Amemura, A., and Futai, M. (1988).J. Biol. Chem. 263, 17437–17442.
Mendel-Hartvig, J., and Capaldi, R. A. (1991a).Biochemistry 30, 1278–1284.
Mendel-Hartvig, J., and Capaldi, R. A. (1991b).Biochemistry 30, 10987–10991.
Skakoon, E. N., and Dunn, S. D. (1993).Arch. Biochem. Biophys. 302, 272–278.
Tang, C., and Capaldi, R. A. (1996).J. Biol. Chem. 271, 3018–3024.
Turina, P., and Capaldi, R. A. (1994a).J. Biol. Chem. 269, 13465–13471.
Turina, P., and Capaldi, R. A. (1994b).Biochemistry 33, 14275–14280.
Watts, S. D., Zhang, Y., Fillingame, R. H., and Capaldi, R. A. (1995).FEBS Lett. 368, 235–238.
Watts, S. D., Tang, C., and Capaldi, R. A. (1996).J. Biol. Chem. 271, in press.
Wilkens, S., and Capaldi, R. A. (1994)Biol. Chem. Hoppe-Seylers 375, 43–51.
Wilkens, S., Dahlquist, F., McIntosh, L. P., Donaldson, L. W., and Capaldi, R. A. (1995).Nature Struct. Biol. 2, 961–967.
Zhang, Y., and Fillingame, R. H. (1995).J. Biol. Chem. 270, 24609–24614.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Capaldi, R.A., Aggeler, R., Wilkens, S. et al. Structural changes in the γ and ε subunits of theEscherichia coli F1F0-type ATPase during energy coupling. J Bioenerg Biomembr 28, 397–401 (1996). https://doi.org/10.1007/BF02113980
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02113980