Abstract
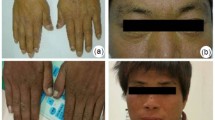
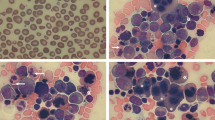
Ferrochelatase [heme synthase, protoheme ferrolyase (EC 4.99.1.1)], the terminal enzyme of the heme biosynthetic pathway, catalyzes the incorporation of ferrous ion into protoporphyrin IX to form protoheme IX. The genes and cDNAs for ferrochelatase from mammals and microorganisms have been isolated. The gene for human ferrochelatase has been mapped to chromosome 18q 21.3 and consists of 11 exons with a size of about 45 kilodaltons. The induction of ferrochelatase expression occurs during erythroid differentiation, and can be attributed to the existence of the promoter sequences of erythroid-related genes. Analysis of the ferrochelatase gene in patients with erythropoietic protoporphyria, an inherited disease caused by ferrochelatase defects, revealed that molecular anomalies of ferrochelatase from 11 patients were found in 9 patients as autosomal dominant type, and 2 patients as recessive type. Diversity of the mutations of the ferrochelatase gene is also briefly described.
Similar content being viewed by others
References
Beru, N., and Goldwasser, E. (1985).J. Biol. Chem. 260, 9251–9257.
Bloomer, J. R., Bloomer, D. A., and Mahoney, M. J. (1977).J. clin. Invest. 60, 1354–1361.
Bloomer, J. R., Morton, K. O., Reuter, R. J., and Ruth (1982).Am. J. Hum. Genet. 34, 322–330.
Bonkowsky, H. L., Bloomer, J. R., Ebert, P. S., Mahoney, M. J. (1975).J. Clin. Invest. 56, 1139–1148.
Bottomley, S. S., Tanaka, M., and Everett, M. A. (1975).J. Labo. Clin. Med. 86, 126–131.
Boulechfar, S., Lamoil, J., Montagutelli, X., Guenet, J.-L., Deybach, J.-C., Nordmann, Y., Dailey, H., Grandchamp, B., and de Verneuil, H. (1993).Gemonics 16, 645–648.
Brenner, D. A., Didier, J. M., Frasier, F., Christensen, S. R., Evans, G. A., and Dailey, H. A. (1992).Am. J. Hum. Genet. 50, 1203–1210.
Chan, R. F. F., Schulman, M., and Ponka, P. (1993).Biochem. J. 292, 343–349.
Chretien, S., Dubart, A., Beaupain, D., Raich, N., Grandchamp, B., Rosa, J., Gossens, M., and Romeo, P.-H. (1988).Proc. Natl. Acad. Sci. USA 85, 6–10.
Dailey, H. A., Sellers, V. M., and Dailey, T. A. (1994).J. Biol. Chem. 269, 390–395.
Fadigan, A., and Dailey, H. A. (1987).Biochem. J. 243, 419–424.
Ferreira, G., Franco, R., Lloyd, S. G., Pereira, R., Moura, I., Moura, J. J. G., Huynh, B. H. (1994).J. Biol. Chem. 269, 7062–7065.
Fujita, H., and Nagai, T. (1993).Seikagaku (Review article),65, 8–24.
Fujita, H., Yamamoto, M., Yamagami, Y., Hayashi, N., Bishop, T. R., de Verneuil, H., Yoshinaga, T., Shibahara, S., Morimoto, R., and Sassa, S. (1991).Biochim. Biophys. Acta 1090, 311–316.
Fukuda, Y., Fujita, H., Taketani, S., and Sassa, S. (1993a).Bri. J. Haematol. 83, 480–483.
Fukuda, Y., Fujita, H., Taketani, S., and Sassa, S. (1993b).Bri. J. Haematol. 85, 670–675.
Furukawa, T., Kohno, H., Tokunaga, R., and Taketani, S. (1995).Biochem. J. in press.
Humphries, R. K., Ley, T. J., Anabnou, N. P., Baur, A. W., and Nienhuis, A. W. (1984).Blood,64, 23–32.
Igarashi, K., Kataoka, K., Itoh, K., Hayashi, N., Nishizawa, W., Yamamoto, M. (1994).Nature 367, 568–572.
Kadowaki, T., Kadowaki, H., and Taylor, S. I. (1990).Proc. Natl. Acad. Sci. USA 87, 658–662.
Kappas, A., Sassa, S., Galbraith, R. A., and Nordmann, Y. (1989).In the Metabolic Basis of Inherited Disease (Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D. eds.), McGraw-Hill, New York, pp 1305–1365.
Klausner, R. D., Rouault, T. A., and Harford, J. B. (1993).Cell 72, 19–28.
Kohno, H., Furukawa, T., Yoshinaga, T., Tokunaga, R., and Taketani, S. (1993).J. Biol. Chem. 268, 21359–21363.
Labee-Bois, R. (1990).J. Biol. Chem. 265, 7278–7283.
Lamoril, J., Boulechfar, S., de Verneuil, H., Grandchamp, B., Nordmann, Y., and Deybach, J.-C. (1991).Biochem. Biophys. Res. Commun. 181, 594–599.
Manugs, I. A., Jarrett, A., Prankert, T. A. J., and Rimington, C. (1961).Lancet ii, 448–451.
Mignotte, V., Wall, L., de Boer, E., Grosveld, F., and Romeo, P.-H. (1980a).Nucleic. Acids Res. 17, 37–54.
Mignotte, V., Eleouet, J. F., Raich, N., and Romeo, P.-H. (1989b).Proc. Natl. Acad. Sci. USA 86, 6548–6552.
Nakahashi, Y., Taketani, S., Sameshima, Y., and Tokunaga, R. (1990a).Biochim. Biophys. Acta 1037, 321–327.
Nakahashi, Y., Taketani, S., Okuda, M., Inoue, K., and Tokunaga, R. (1990b).Biochem. Biophys. Res. Commun. 173, 748–755.
Nakahashi, Y., Fujita, H., Taketani, S., Ishida, N., Kappas, A., and Sassa, S. (1992).Proc. Natl. Acad. Sci. USA 89, 281–285.
Nakahashi, Y., Miyazaki, H., Kadota, Y., Naitoh, Y., Inoue, K., Yamamoto, M., Hayashi, N., and Taketani, S. (1993a).Hum. Genet. 91, 303–306.
Nakahashi, Y., Miyazaki, H., Kadota, Y., Naitoh, Y., Inoue, K., Yamamoto, M., Hayashi, N., and Taketani, S. (1993b).Hum. Mol. Genet. 2, 1069–1070.
Orkin, S. H. (1990).Cell 63, 665–672.
Peters, L. L., Andrews, N. C., Eicher, R. S., Davidson, M. B., Orkin, S. H., and Lux, S. E. (1993a).Nature 362, 768–770.
Peters, L. L., Bishop, T. R., and Andrews, N. C. (1993b).Blood 82, 179a (suppl.).
Philipson, S., Talbot, D., Fraser, P., and Grosveld, F. (1990).EMBO J. 9, 2159–2169.
Rutherford, T., Thompson, G. G., and Moore, M. R. (1979).Proc. Natl. Acad. Sci. USA 76, 833–836.
Sarkany, R. P. E., Alexander, G. J. M. A., and Cox, T. M. (1994a).Lancet,343, 1394–1396.
Sarkany, R. P. E., Whitcombe, D. M. and Cox, T. M. (1994b).J. Invest. Dermatol. 102, 481–484.
Sassa, S. (1976).J. Exp. Med. 143, 305–315.
Sassa, S. (1980).In vivo and in vitro Erythropoesis: The Friend System (Rosi, G. B. ed.), Elsevier/North Holland Biomedical Press, New York, pp 219–228.
Sassa, S., and Urabe, A. (1979).Proc. Natl. Acad. Sci. USA 76, 5321–5325.
Sassa, S., Zalar, G. L., Poh-Fitzpatrick, M. B., Anserson, K. E., and Kappas, A. (1982).J. Clin. Invest. 69, 809–815.
Schneider-Yin, X., Schaefer, B. W., Moehr, P., Burg, G., and Minder, E. I. (1994a).Hum. Genet. 93, 711–713.
Schneider-Yin, X., Taketani, S., Schaefer, B., and Minder, E. I. (1994b).Lancet 344, 337.
Taketani, S. (1993).Tohuku J. Exp. Med. (Review Article)171, 1–20.
Taketani, S., Nakahashi, Y., Osumi, T., and Tokunaga, R. (1990).J. Biol. Chem. 265, 24637–24641.
Taketani, S., Inazawa, J., Nakahashi, Y., Abe, T., and Tokunaga, R. (1992).Eur. J. Biochem. 205, 217–222.
Todd, D. J., Hughes, A. E., Ennis, K. T., Ward, A. J., Burrows, D., and Norman, C. N. (1993).Hum. Mol. Genet. 2, 1495–1496.
Tutois, S., Montagutelli, X., de Siva, V., Jouault, H., Rouyer-Fessard, P., Leroy-Viard, K., Guenet, J.-L., Nordmann, Y., Beuzaed, Y., and Deybach, J.-C. (1991).J. Clin. Invest. 88, 1730–1736.
Urlaub, G., Mitchell, P. J., Ciudad, C. J., and Chasin, K. A. (1989).Mol. Cell. Biol. 9, 2868–2880.
Wang, X., Poh-Fitzpatrick, M., Carriero, D., Ostasiewicz, L., Chen, T., Taketani, S., and Piomelli, S. (1993a).Biochim. Biophys. Acta 1181, 198–200.
Wang, X., Poh-Fitzpatrick, M., Takctani, S., Chen, T., and Piomelli, S. (1993b).Biochim. Biophys. Acta 1225, 187–190.
Whitcombe, D. M., Carter, N. P., Albertson, D. G., Smith, S. J., Rhodes, D. A., and Cox, T. M. (1991).Hum. Mol. Genet. 2, 826.
Whitcombe, D. M., Albertson, D. G., and Cox, T. M. (1994).Genomics 20, 482–486.
Yamamoto, M., Lim, K.-C., Nagai, T., Furuyama, K., and Engel, J. D. (1994).In Regulation of Heme Protein Synthesis (Fujita, H. ed.) Alpha Med Press, Dayton, Ohio pp 11–26.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taketani, S., Fujita, H. The ferrochelatase gene structure and molecular defects associated with erythropoietic protoporphyria. J Bioenerg Biomembr 27, 231–238 (1995). https://doi.org/10.1007/BF02110038
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02110038