Summary
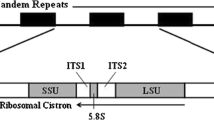
DNA sequence analysis and the localization of the 5′ and 3′ termini by S1 mapping have shown that the mitochondrial (mt) small subunit rRNA coding region fromPodospora anserina is 1980 bp in length. The analogous coding region for mt rRNA is 1962 bp in maize, 1686 bp inSaccharomyces cerevisiae, and 956 bp in mammals, whereas its counterpart inEscherichia coli is 1542 bp. TheP. anserina mt 16S-like rRNA is 400 bases longer than that fromE. coli, but can be folded into a similar secondary structure. The additional bases appear to be clustered at specific locations, including extensions at the 5′ and 3′ termini. Comparison with secondary structure diagrams of 16S-like RNAs from several organisms allowed us to specify highly conserved and variable regions of this gene. Phylogenetic tree construction indicated that this gene is grouped with other mitochondrial genes, but most closely, as expected, with the fungal mitochondrial genes.
Similar content being viewed by others
References
Brosius J, Dull TJ, Noller HF (1980) Complete nucleotide sequence of a 23S ribosomal RNA gene fromEscherichia coli. Proc Natl Acad Sci USA 77:201–204
Brown TA, Waring RB, Scazzocchio C, Davies RW (1985) TheAspergillus nidulans mitochondrial genome. Curr Genet 9: 113–117
Brown WM, George MJ, Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76: 1967–1971
Browning KS, RajBhandary UL (1982) Cytochrome oxidase subunit III gene inNeurospora crassa mitochondria. J Biol Chem 257:5253–5256
Chomyn A, Mariottini P, Cleeter MWJ, Ragan CI, Matsuno-Yagi A, Hatifi Y, Doolittle RF, Attardi G (1985) Six unidentified reading frames of human mitchondrial DNA encode components of the respiratory chain NADH dehydrogenase. Nature 314:592–597
Cummings DJ, Belcour L, Grandchamp C (1979) Mitochondrial DNA fromPodospora anserina I. Isolation and characterization. Mol Gen Genet 171:229–238
Cummings DJ, MacNeil IA, Domenico J, Matsuura ET (1985) Excision-amplification of mitochondrial DNA during senescence inPodospora anserina. J Mol Biol 185:659–680
Cummings DJ, Domenico JM, Nelson J, Michel F (1988) Evolution in process. J Mol Evol (submitted)
Dron M, Rahire M, Rochaix JD (1982) Sequences of the chloroplast 16S rRNA gene and its surrounding regions ofChlamydomonas reinhardtii. Nucleic Acids Res 10:7609–7620
Dujon B (1983) Mitochondrial genes, mutants and maps: a review. In: Schweyen RJ, World K, Kaudewit (eds) Mitochondria 1983. Walter de Gruyter & Co, Berlin, pp 1–24
Elwood HJ, Olsen GJ, Sogin ML (1985) The small subunit RNA gene sequence from the hypotrichous ciliatesOxytricha nova andStylonychia pustulata. Mol Biol Evol 2:399–410
Graf L, Roux E, Stutz E, Kossel H (1982) Nucleotide sequence ofEuglena gracilis chloroplast gene coding for the 16S rRNA: homologies toE. coli andZea mays chloroplast 16Sr RNA. Nucleic Acids Res 10:6369–6381
Gray MW, Doolittle WF (1982) Has the endosymbiont hypothesis been proven? Microbiol Rev 46:1–42
Green CJ, Stewart GC, Hollis MA, Vold BS, Bott KF (1985) Nucleotide sequence ofBacillus subtilis ribosomal RNA operon. Gene 37:261–266
Gupta R, Broccoli A, Woese CR (1983) Sequence of the 16S ribosomal RNA fromHalobacterium volcanii an archebacterium. Science 221:656–659
Gutell RR, Weiser B, Woese CR, Noller HF (1985) Comparative anatomy of 16S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol 32:155–216
Gutell RR, Noller HF, Woese CR (1986) Higher order structure in ribosomal RNA. EMBO J 5:1111–1113
Hall LMC, Madden BEH (1980) Nucleotide sequence through the 18S–28S intergene region of a vertebrate ribosomal transecription unit. Nucleic Acids Res 8:5993–6005
Jacob WF, Santer M, Dahlberg AE (1987) A single base change in the Shine-Dalgarno region of 16S rRNA ofE. coli affects translation of many proteins. Proc Natl Acad Sci USA 84: 4757–4761
Jamet-Vierny C, Begel O, Belcour L (1984) A 20α103 base mosaic gene identified on the mitochondrial chromosome ofPodospora anserina. J Biochem 143:389–394
Kochel HG, Kuntzel H (1981) Nucleotide sequence of theAspergillus nidulans mitochondrial gene coding for the small ribosomal subunit RNA homology toE. coli 16S rRNA. Nucleic Acids Res 9:5689–5696
Kochel HG, Kuntzel H (1982) Mitochondrial L-rRNA fromAspergillus nidulans: potential secondary structure and evolution. Nucleic Acids Res 10:4795–4801
Kück U, Kappelhoff B, Esser K (1985) Despite mtDNA polymorphism the mobile intron (pIDNA) or the COI gene is present in ten different races ofPodospora anserina. Curr Genet 10:59–67
Li M, Tzagoloff A, Underbrink-Lyon K, Martin NC (1982) Identification of the paromomycin-resistance mutation in the 15S rRNA gene of yeast mitochondria. J Biol Chem 257:5921
Masters BS, Stohl LL, Clayton DA (1987) Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T1. Cell 51:89–99
Maxam AM, Gilbert W (1980) Sequencing DNA by labeling the end and breaking at bases: DNA segmetns, end-labels, cleavage reactions; polyacrylamide gels and strategies. Methods Enzymol 65:499–560
McCarrol R, Olsen GJ, Stahl YD, Woese CR, Sogin ML (1983) Nucleotide sequence of theDictyostelium discoideum small-subunit ribosomal ribonucleic acid inferred from the gene sequence. Evolutionary implications. Biochemistry 22:5858–5868
Michel F, Jacquier A, Dujon B (1982) Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochemie 64:867–881
Morelli G, Macino G (1984) Two intervening sequences in the ATPase subunit 6 gene ofNeurospora crassa. A short intron (93 base-paris) and a long intron that is stable after excision. J Mol Biol 178:491–507
Netzker R, Kochel HG, Basak N, Kuntzel H (1982) Nucleotide sequence ofAspergillus nidulans mitochondrial genes coding for ATPase subunit 6, cytochrome oxidase subunit 3, seven unidentified proteins, four tRNA's and L-rRNA. Nucleic Acids Res 10:4783–4794
Noller HF (1984) Structure of ribosomal RNA. Annu. Rev Biochem 53:119–162
Prince JB, Taylor BH, Thurlow DL, Ofengard J, Zimmerman RA (1982) Covalent cross-linking of tRNAval to 16S RNA at the ribosomal P site: identification of cross-linked residues. Proc Natl Acad Sci USA 79:5450–5454
Schnare MN, Heinonen TYK, Young PG, Gray MW (1986) A discontinuous small subunit ribosomal RNA inTetrahymena pyriformis mitochondria. J Biol Chem 261:5187–5193
Schwartz A, Kossel H (1979) Sequencing of the 3′ terminal region of a 16S rRNA gene fromZea mays chloroplast reveals homology withE. coli 16S RNA. Nature 279:520–522
Schwartz A, Kossel H (1980) The primary structure of 16S rRNA fromZea mays chloroplast is homologous toE. coli 16S rRNA. Nature 283:739–742
Seilhamer JJ, Olsen GJ, Cummings DJ (1984) Paramecium mitochondrial genes I. Small subunit rRNA gene sequence and microevolution. J Biol Chem 259:5167–5172
Shine J, Dalgarno L (1974) The 3′ terminal sequence ofEscherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA 71:1342–1346
Sor F, Fukuhara H (1983) Compete DNA sequence coding for the large ribosomal RNA of yeast mitochondrial. Nucleic Acids Res 11:339–348
Spencer DF, Schnare MN, Gray MW (1984) Pronounced structural similarities between the small subunit ribosomal RNA genes of wheat mitochondria andEscherichia coli. Proc Natl Acad Sci USA 81:493–497
Tohdoh N, Sugiura M (1982) The complete nucleotide sequence of a 16S ribosomal RNA gene from tobacco chloroplasts. Gene 17:213–218
Tomioka N, Suguira M (1983) The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green algaAnacystis nidulans. Mol Gen Genet 191:46–50
Woese CR (1987) Bacterial evolution. Microbiol Rev 47:621–669
Woese CR, Gutell R, Gupta R, Noller HF (1983) Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev 47:621–669
Wright RM, Cummings DJ (1983a) Mitochondrial DNA fromPodospora anserina IV. The large ribosomal RNA gene contains two long intervening sequences. Curr Genet 7:151–157
Wright RM, Cummings DJ (1983b) Transcription of a mitochondrial plasmid during senescence inPodospora anserina. Curr Genet 7:457–464
Wright RM, Horrum NA, Cummings DJ (1982a) Are mitochondrial structural genes selectively amplified during senescence ofPodospora anserina? Cell 29:505–515
Wright RM, Laping JL, Horrum MA, Cummings DJ (1982b) Mitochondrial DNA fromPodospora anserina III. Cloning, physical mapping and localization of the ribosomal RNA genes. Mol Gen Genet 185:56–64
Yang D, Oyaizu Y, Oyaizu H, Olsen GJ, Woese CR (1985) Mitochondrial origins. Proc Natl Acad Sci USA 82:4443–4447
Yin S, Heckman J, RajBhandary UL (1981) Highly conserved GC-rich palindromic DNA sequences flank, tRNA genes inNeurospora crassa mitochondria. Cell 26:325–332
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cummings, D.J., Domenico, J.M., Nelson, J. et al. DNA sequence, structure, and phylogenetic relationship of the small subunit rRNA coding region of mitochondrial DNA fromPodospora anserina . J Mol Evol 28, 232–241 (1989). https://doi.org/10.1007/BF02102481
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02102481