Abstract
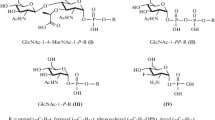
Mycosides are glycosides of p-phenols with a branched glycolic chain the hydroxyl functions of which are esterified with normal and branched chain fatty acids. They differ by the nature and number of sugar molecules which are attached to the phenolic hydroxyl group of the aglycones (Fig. 15).
The structures of the aglycones of mycoside A (isolated fromM. kansasii) and of mycoside B (isolated from bovine strains) are established by oxidative degradation and confirmed by mass spectrometry.
The phenol-glycols A and B differ by the length of the aliphatic glycolic chains. Each aglycone is a mixture of homologues (the fatty acids are homologues and the phenol-glycols are homologues).
The scheme of incorporation of propionic acid into phenol-glycols A and B is the same as the one which has been established for phthiocerol.
Résumé
Les mycosides sont des glycosides de p-phénols ayant une longue chaîne glycolique ramifiée dont les fonctions hydroxyles sont estérifiées par des acides gras normaux et ramifiés. Ils diffèrent par la nature et le nombre des molécules de sucres qui sont fixées sur l'hydroxyle phénolique des aglycones (Fig. 15).
Les structures des aglycones du mycoside A (isolé deM. kansasii) et du mycoside B (isolé de souches bovines) ont été établies par dégradation oxydative et confirmées par spectrométrie de masse.
Les phénol-glycols A et B diffèrent par la longeur des chaînes glycoliques aliphatiques. Chacun des aglycones A et B est un mélange de substances homologues (les acides gras sont des homologues et les phénol-glycols sont des homologues).
L'acide propionique radioactif est incorporé dans les phénol-glycols A et B selon un schéma analogue à celui du phtiocérol. La phénylalanine n'est pas incorporée dans le noyau aromatique des mycosides.
Similar content being viewed by others
Bibliographie
Ahlquist, L., Ryhage, R., Stenhagen, E., Sydow, E. von: Studies on phthiocerol. I. Mass spectromatric investigation of phthiocerol and certain structurally related compounds. Ark. för Kemi14, 211 (1959)
Asselineau, C., Asselineau, J., Ryhage, R., Ställberg-Stenhagen, S., Stenhagen, E.: Synthesis of (-)-Methyl 2D, 4D, 6D-trimethylnonacosanoate and identification of C32-Mycocerosio acid as a 2,4,6,8-tetramethyloctacosanoic acid. Acta chem. scand.13, 822 (1959).
Bernfeld, P.: Biogenesis of natural compounds. Pergamon Press 1963.
Bickel, H., Schmid, H., Karrer, P.: Zur Kenntnis des Fluorocurins und Mavacurins. 15. Mitteilung über Curare-Alkaloide aus Calebassen. Helv. chim. Acta38, 649 (1955).
Bohm, B. A.: Shikimic acid (3,4,5-trihydroxy-1-cyclohexene-1-carboxylic acid). Chem. Rev.65, 435 (1965).
Budzikiewicz, H., Djerassi, C., Williams, D. H.: Interpretation of mass spectra of organic compounds. San Francisco: Holden-Day Inc. 1964.
Coggeshall, N. O., Glessner, A. S. Jr.: Ultraviolet absorption study of the ionization of substituted phenols in ethanol. J. Amer. chem. Soc.71, 3151 (1949).
Demarteau-Ginsburg, H., Lederer, E.: Sur la structure chimique du mycoside B. Biochim. biophys. Acta (Amst.)70, 442 (1963).
Drayson, F. K., Lewis, J. W., Polgar, N.: Experiments relating to phthiocerol. Part III. Degradative studies of a C11 oxidation product of phthiocerol. J. chem. Soc. 430 (1958).
Gastambide-Odier, M., Delaumeny, J. M., Lederer, E.: Biosynthesis of phthiocerol: Incorporation of methionine and propionic acid. Chem. and Ind. 1285 (1963).
—— —— —— Biosynthèse de l'acide C32-mycocérosique: Incorporation d'acide propionique. Biochem. biophys. Acta (Amst.)70, 670 (1963).
—— Smith, D. W., Koevoet, A. L., Randall, H. M.: Comparison of the native lipids of atypical acid-fast bacilli with the lipids of known mycobacterial types. Amer. Rev. Tuberc.75, 843 (1957).
Hauser, G., Karnovsky, M. L.: Studies on the biosynthesis of L-rhamnose. J. biol. Chem.233, 287 (1958).
Heath, E. C., Roseman, S.: Conversion of D-glucose to L-fucose by Aerobacter cloacae. J. biol. Chem.230, 311 (1958).
Highet, R. J., Highet, P. F.: The characterization of complex phenols by nuclear magnetic resonance spectra. J. Org. Chem.30, 902 (1965).
Kuhn, L. P.: The hydrogen bond. I. Intra- and intermolecular hydrogen bonds in alcohols. J. Amer. chem. Soc.74, 2492 (1952).
MacLennan, A. P., Randall, H. M., Smith, D. W.: The occurrence of methyl ethers of rhamnose and fucose in specific glycolipids of certain Mycobacteria. Biochem. J.80, 309 (1961).
Noll, H.: The chemistry of some native constituents of the purified wax of Mycobacterium tuberculosis. J. biol. Chem.224, 149 (1957).
Ryhage, R., Stenhagen, E.: Mass spectrometry in lipid research. J. Lipid Res.1, 361 (1960).
—— Ställberg-Stenhagen, S., Stenhagen, E.: Studies on phthiocerol. II. The nature of the acidic products obtained on oxidation of phthiocerol by chromic acid. Ark. för Kemi14, 247 (1959).
Smith, D. W., Randall, H. M., Gastambide-Odier, M., Koevoet, A. L.: The characterization of mycobacterial strains by the composition of their lipide extracts. Ann. N.Y. Acad. Sci.69, 145 (1957).
—— —— MacLennan, A. P., Putney, R. K., Rao, S. V.: Detection of specific lipids in Mycobacteria by infrared spectroscopy. J. Bact.79, 217 (1960).
—— —— —— Lederer, E.: Mycosides: a new class of type-specific glycolipids of Mycobacteria. Nature (Lond.)186, 887 (1960).
—— Harrell, W. K., Randall, H. M.: Correlation of biologic properties of strains of Mycobacterium with their infrared spectrums. III. The differentiation of bovine and human varieties of M. tuberculosis by means of infrared spectrums. Amer. Rev. Tuberc.69, 505 (1954).
Author information
Authors and Affiliations
Additional information
Ce travail a bénéficié d'une subvention du National Institute of Allergy and Infectious Diseases (U.S. Public Health Service), Grant AI-02838, et d'une subvention du Commissariat à l'Energie Atomique, Saclay, pour l'achat de radioisotopes.
Rights and permissions
About this article
Cite this article
Gastambide-Odier, M., Sarda, P. Contribution à l'étude de la structure et de la biosynthèse de glycolipides spécifiques isolés de mycobactéries: les mycosides A et B. Pneumologie 142, 241–255 (1970). https://doi.org/10.1007/BF02095222
Issue Date:
DOI: https://doi.org/10.1007/BF02095222