Abstract
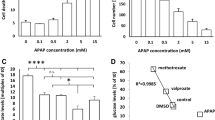
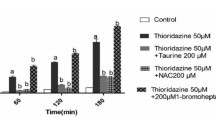
The aims of this study were to investigate the roles of iron as a catalyst in reactive oxygen metabolite-mediated cellular injury and of the endogenous antioxidant defenses against acetaminophen-induced cytotoxicity in cultured rat hepatocytes. Hepatocytes were isolated and cultured from either 3-methylcholanthrene-treated or untreated rats. Cytotoxicity was evaluated by measuring51Cr and lactate dehydrogenase release. Acetaminophen caused dose-dependent cytotoxicity in 3-methlycholanthrene-treated, but not untreated, cells. There was a good correlation between51Cr and lactate dehydrogenase release values. Pretreatment with both diethyl maleate, which covalently binds glutathione as catalyzed by glutathione-S-transferase, and bis(chloroethyl)-nitrosourea, an inhibitor of glutathione reductase, enhanced acetaminophen-induced cytotoxicity. Inhibition of endogenous catalase activity by pretreatment with aminotriazole did not affect acetaminophen-induced cellular damage. Addition of exogenous catalase failed to protect against acetaminophen-induced cytotoxicity. Preincubation with both deferoxamine, a ferric iron chelator, and phenanthroline, a ferrous iron chelator, diminished acetaminophen-induced cytotoxicity. These results indicate that iron is crucial in mediating acetaminophen-induced cytotoxicity and that the glutathione redox cycle, but not catalase, plays a critical role in the endogenous defenses against acetaminophen-induced cellular damage in cultured rat hepatocytesin vitro.
Similar content being viewed by others
References
Boyd EM, Bereczky GM: Liver necrosis from paracetamol. Br J Pharmacol 26:606–614, 1966
Clark R, Borirakchanyavat V, Davidson AR, Thompson RPH, Widdop B, Goulding R, Williams R: Hepatic damage and death from overdose of paracetamol. Lancet 1:66–69, 1973
Prescott LF, Wright N, Roscoe P, Brown SS: Plasma-paracetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet 1:519–522, 1971
Boyer TD, Rouff SC: Acetaminophen-induced hepatic necrosis and renal failure. JAMA 218:440–441, 1971
Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB: Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187:185–194, 1973
Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB: Acetaminophen-induced hepatic necrosis. II. Role of covalent bindingin vivo. J Pharmacol Exp Ther 187:195–202, 1973
Potter WZ, Davis DC, Mitchell JR, Jollow DJ, Gillette JR, Brodie BB: Acetaminophen-induced hepatic necrosis. III. Cytochrome P-450-mediated covalent bindingin vitro. J Pharmacol Exp Ther 187:203–210, 1973
Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB: Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217, 1973
Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H: Acetaminophen-induced hepatic injury: Protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther 16:676–684, 1974
Davis DC, Potter WZ, Jollow DJ, Mitchell JR: Species differences in hepatic glutathione depletion: Covalent binding and hepatic necrosis after acetaminophen. Life Sci 14:2099–2109, 1974
Rosen GM, Singletaly WV Jr, Rauckman EJ, Killenberg PG: Acetaminophen hepatotoxicity. An alternative mechanism. Biochem Pharmacol 32:2052–2059, 1983
Wendel A, Feuerstein S: Drug-induced lipid peroxidation in mice -I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol 30:2513–2520, 1981
Wendel A, Jaeschke H: Drug-induced lipid peroxidation in mice -II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol 31:3601–3605, 1982
Gerson RJ, Casini A, Gilfor D, Serroni A, Farber JL: Oxygen-mediated cell injury in the killing of cultured hepatocytes by acetaminophen. Biochem Biophys Res Commun 126:1129–1137, 1985
Kyle ME, Miccadei S, Nakae D, Farber JL: Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun 149:889–896, 1987
Haber F, Weiss J: The catalytic decomposition of hydrogen peroxide by iron salt. Proc R Soc London A Math Phys Sci 147:332–351, 1934
Halliwell B: Superoxide-dependent formation of hydroxyl radicals in the presence of iron salt is a feasible source of hydroxyl radicalsin vivo. Biochem J 205:461–462, 1982
MacCord JM, Day ED: Superoxide-dependent production of hydroxyl radical catalyzed by an iron-EDTA complex. FEBS Lett 86:139–142, 1978
Ito Y, Hiraishi H, Razandi M, Terano A, Harada T, Ivey KJ: Role of cellular superoxide dismutase against reactive oxygen metabolite-induced cell damage in cultured rat hepatocytes. Hepatology 16:247–254, 1992
Selgen PO: Preparation of isolated rat liver cells.In Methods in Cell Biology, Vol 13, DM Prescott (ed). New York, Academic Press, 1976, pp 29–83
Tanaka K, Sato M, Tomita Y, Ichihara A: Biochemical studies on liver functions in primary cultured hepatocytes of adult rats. I. Hormonal effects on cell viability and protein synthesis. J Biochem 84:937–946, 1978
Omura T, Sato R: The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378, 1964
Lake BG: Preparation and characterisation of microsomal fractions for studies on xenobiotic metabolism.In Biochemical Toxicology. A Practical Approach. K Snell, B Mullock (eds). Oxford, IRL Press, 1987, pp 183–215
Woolson RF: Statistical Methods for the Analysis of Biomedical Data. New York, John Wiley & Sons, 1987
Astrom A, Maner S, Depierre J: Induction of cytochrome P-450 and related drug-metabolizing activities in the livers of different rodent species by 2-acethylaminofluorene or by 3--methylcholanthrene. Biochem Pharmacol 35:2703–2713, 1986
Chance B, Sies H, Boveris A: Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605, 1979
Kamath NS, Satyamoorthy K, Chitnis MP, Advani SH: 1,10-Phenanthroline potentiates cytotoxicity of hydroxyurea in human chronic myeloid leukemia cells. Oncology 46:193–197, 1989
Porter JB, Gyparaki M, Burke LC, Huehns ER, Sarpong P, Saez V, Hider RC: Iron mobilization from hepatocyte monolayer cultures by chelators: The importance of membrane permeability and the iron-binding constant. Blood 72:1497–1503, 1988
Kontoghiorghes GJ, Piga A, Hoffbrand AV: Cytotoxic effects of the lipophilic iron chelator omadine. FEBS Lett 204:208–212, 1986
Younes M, Sause C, Siegers CP: Effect of deferrioxamine and diethyldithiocarbamate on paracetamol-induced hepato-and nephrotoxicity. The role of lipid peroxidation. J Appl Toxicol 8:261–265, 1988
Freeman BA, Crapo JD: Biology of disease. Free radicals and tissue injury. Lab Invest 47:412–426, 1982
Flohe L: Glutathine peroxidase: Fact and fiction.In Oxygen Free Radicals and Tissue Damage. G Cohen, RA Greenwald (eds). Amsterdam, Excerpta Medica, 1979, pp 95–122 [Ciba Foundation Symposium 65 (new series)]
Freeman BA, Young SL, Crapo JD: Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem 258:12534–12542, 1983
Buckley BJ, Tanswell AK, Freeman BA: Liposome-mediated augmentation of catalase in alveolar type II cells protects against H2O2 injury. J Appl Physiol 63:359–367, 1987
Author information
Authors and Affiliations
Additional information
This work was supported by the Medical Research Service of the Veterans Affairs.
Rights and permissions
About this article
Cite this article
Ito, Y., Suzuki, Y., Ogonuki, H. et al. Role of iron and glutathione redox cycle in acetaminophen-induced cytotoxicity to cultured rat hepatocytes. Digest Dis Sci 39, 1257–1264 (1994). https://doi.org/10.1007/BF02093791
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02093791