Abstract
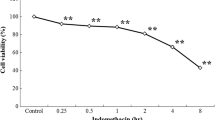
In the current study, the role of endonuclease activity in calcium ionophore A23187-induced gastric mucosal cellular disruption was examined using rabbit gastric mucosal cells. Cell integrity was assessed using trypan blue dye exclusion and Alamar blue dye absorbance. Ionophore A23187 (1.6–25 µM) induced a concentration-dependent decrease in dye exclusion and cell metabolism in cells suspended in a medium containing Ca2+ (2 mM), while no such effect was observed in cells incubated in the absence of extracellular Ca2+. Cells that were pretreated with the endonuclease inhibitors aurintricarboxylic acid (ATCA; 0.2 or 0.5 mM or Zn2+; 0.01 and 0.1 mM) exhibited significant reduction in the total extent of cell injury when incubated with A23187 in the presence of Ca2+. DNA fragmentation as assessed by measurement of [3H]thymidine liberation or gel electrophoresis was increased in response to ionophore A23187 (12.5 or 25 µM) treatment. A minimal degree of fragmentation was observed when cells were suspended in a Ca2+-free medium or incubated in the presence of ATCA or Zn2+. Addition of ethanol (8% w/v) induced a significant increase in cell injury, which was not affected by either removal of extracellular Ca2+ or ATCA pretreatment. Furthermore, treatment with the antioxidants catalase (50 µg/ml) or 2′,2′-dipyridyl (2 mM) reduced ionophore-induced cell injury but did not reduce the extent of DNA fragmentation. These data suggest that sustained increases in intracellular Ca2+ result in increased endonuclease activity in gastric mucosal cells, leading to extensive DNA lysis and cell damage. Ethanol-induced cell damage does not involve Ca2+ influx and therefore is not mediated by endonuclease activation. Furthermore, sustained increases in cellular Ca2+ may also mediate their effects via formation of reactive oxygen metabolites, but this mechanism of cell damage does not appear to involve DNA fragmentation.
Similar content being viewed by others
References
Farber JL: The role of calcium in cell killing. Life Sci 29:1289–1295, 1981
Farber JL: The role of calcium of calcium ions in toxic cell injury. Environ Health Perspect 84:107–111, 1990
Leonard JP, Saltpeter MM: Calcium mediated myopathy at neuromuscular junctions of normal and dystrophic muscle. Exp Neurol 76:121–138, 1982
Pasternak CA: Effect of pore formers on intracellular calcium. Cell Calcium 7:387–397, 1986
Nicotera P, Hartzell P, Davis G, Orrenius S: The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett 209:1309–1344, 1986
Nicotera P, Hartzell P, Baldi C, Svenson S-A, Bellomo G, Orrenius S: Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+. J Biol Chem 261:14628–14635, 1986
Mirabelli F, Salis A, Vairetti M, Bellomo G, Thor H, Orrenius S: Cytoskeletal alterations in human platelets exposed to oxidative stress are mediated by oxidative and Ca+-dependent mechanisms. Arch Biochem Biophys 270:478–488, 1989
Dziki AJ, Batzri S, Harmon JW, Molloy M: Cellular hypercalcemia is an early event in deoxycholate injury of rabbit mucosal cells. Am J Physiol 269(Gastrointest Liver Physiol 32):G287-G296, 1995
Tepperman BL, Soper BD: Calcium-mediated damage to rabbit gastric mucosal cells: Modulation by nitric oxide. Eur J Pharmacol 293:259–266, 1995
Ota S, Tsukahara H, Terano H, Hata Y, Hiraishi H, Mutoh H, Sugimoto T: Protective effects of tauroursodeoxycholate against chenodeoxycholate-induced damage to cultured rabbit gastric cells. Dig Dis Sci 36:409–416, 1991
Tepperman BL, Soper BD: Effect of extracellular Ca2+ on indomethacin-induced injury to rabbit dispersed gastric mucosal cells. Can J Physiol Pharmacol 72:63–69, 1994
McConkey DJ, Hartzell P, Duddy SK, Hakansson H, Orrenius S: 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science 242:256–295, 1989
Lohmann RD, Beyersmann D: Effects of zinc and calcium on apoptotic DNA fragmentation in isolated bovine liver nuclei. Environ Health Perspect 102(suppl 3):269–271, 1994
Zhivotovsky B, Cedervall B, Jiang S, Nicotera P, Orrenius S: Involvement of Ca2+ in the formation of high molecular weight DNA fragments in thymocyte apoptosis. Biochem Biophys Res Commun 202:120–127, 1994
Matsubara K, Kubota M, Adachi S, Kuwakado K, Hirota H, Wakazono Y, Akiyama Y, Mikawa H: Different mode of cell death induced by calcium ionophore in human leukemia cell lines: Possible role of constitutive endonuclease. Exp Cell Res 210:19–25, 1994
Valle VG, Fagian MM, Parentoni LS, Meinicke AR, Vercesi AE: The participation of reactive oxygen species and protein permeabilization by calcium plus proxidants. Arch Biochem Biophys 307:1–7, 1993
Wong HM, Tepperman BL: Reduced glutathione modulates Ca2+-mediated damage to rabbit isolated gastric mucosal cells. Am J Physiol 267(Gastrointest Liver Physiol 30):G1-G9, 1994
Ben-Yoseph O, Ross BD: Oxidation therapy: The use of a reactive oxygen species-generating enzyme system for tumor treatment. Br J Cancer 70:1131–1135, 1994
Kim H-R, Rho H-W, Park B-H, Park J-W, Kim J-S, Kim U-H, Chung M-Y: Role of Ca2+ in alloxan-induced pancreaticβ-cell damage. Biochim Biophys Acta 1227:87–91, 1994
Tepperman BL, Tan SY, Whittle BJR: Effects of calcium-modifying agents on integrity of rabbit gastric mucosal cells. Am J Physiol 261(Gastrointest Liver Physiol 24):G119-G127, 1994
McConkey DJ, Hartzell P, Nicotera P, Orrenius S: Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J 3:1843–1849, 1989
Pagé B, Pagé M, Noel C: A new fluorometric assay for cytotoxicity measurementsin vitro. Int J Oncol 3:473–476, 1993
Philippe C, Philippe B, Fouqueray B, Perez J, Lebret M, Baud L: Protection from tumor necrosis factor-mediated cytolysis by platelets. Am J Pathol 143:1713–1723, 1993
Robaye B, Mosselmans R, Fiers W, Dumont JE, Galand F: Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cellsin vitro. Am J Pathol 138:447–453, 1991
Wong HM, Soper BD, Tepperman BL: Role of calcium in thromboxane B2-mediated injury to rabbit gastric mucosal cells. Dig Dis Sci 40:2022–2028, 1995
Mosmann T: Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity. J Immunol Methods 65:55–64, 1983
Nagy LS, Szabo S, Morales RE, Plebani M, Jenkins JM: Identification of subcellular targets and sensitive tests of ethanol-induced damage in isolated rat gastric mucosal cells. Gastroenterology 107:907–914, 1994
Jiang S, Chow SC, Nicotera P, Orrenius S: Intracellular Ca2+ signals activate apoptosis in thymocytes: Studies using the Ca2+-ATPase inhibitor thapsigargin. Exp Cell Res 212:84–92, 1994
Csernansky CA, Canzoniero LMT, Sensi SL, Yu SP, Choi DW: Delayed application of aurintricarboxylic acid reduces glutamate-induced cortical neuronal injury. J Neurosci Res 38:101–108, 1994
Kokileva L: Multi-step chromatin degradation in apoptosis. Int Arch Allergy Immunol 105:339–343, 1994
Walker PR, Weaver VM, Lach B, LeBlanc J, Sikorska M: Endonuclease activities associated with high molecular weight and internucleosomal DNA fragmentation in apoptosis. Exp Cell Res 213:100–106, 1994
Cain K, Inayat-Hussain SH, Wolfe JT, Cohen GM: DNA fragmentation into 200–250 and/or 30–50 kilobase pair fragments in rat liver nuclei is stimulated by Mg2+ alone and Ca2+/Mg2+ but not by Ca2+ alone. FEBS Lett 349:385–391, 1994
Cherner JA, Naik L, Tarnawski A, Brzozowski J, Stachura J, Singh G: Ability of prostaglandin to reduce ethanol injury to dispersed chief cells from guinea pig stomach. Am J Physiol 256(Gastrointest Liver Physiol 19):G704-G714, 1989
Duke RC, Chervenak R, Cohen JJ: Endogenous endonuclease-induced DNA fragmentation: An early event in cell-mediated cytolysis. Proc Natl Acad Sci USA 80:6361–6365, 1983
Patel T, Bronk SF, Gores GJ: Increases in intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest 94:2183–2192, 1994
Author information
Authors and Affiliations
Additional information
This work was supported by a grant from the Medical Research Council of Canada (MT 6426).
Rights and permissions
About this article
Cite this article
Tepperman, B.L., Lush, C.W. & Soper, B.D. Role of endonuclease activity and DNA fragmentation in Ca2+ ionophore A23187-mediated injury to rabbit isolated gastric mucosal cells. Digest Dis Sci 41, 1172–1180 (1996). https://doi.org/10.1007/BF02088234
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02088234