Summary
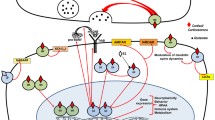
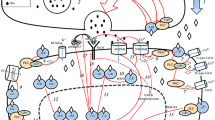
1. The mapping of glucocorticoid receptors (GR) in the rat central nervous system (CNS) has demonstrated their widespread presence in large numbers of nerve and glial cell populations also outside the classical stress regions.
2. The present paper summarizes the evidence that glucocorticoids via GR in the CNS can act as lifelong organizing signals from development to aging. The following examples are given. (a) In the prepubertal and adult offspring, prenatal corticosterone treatment can produce long-lasting changes in striatal dopaminergic communication. (b) In adulthood, the evidence suggests complex regulation by adrenocortical hormones of neurotrophic factors and their receptors in the hippocampal formation. (c) In aging, the strongly GR-immunoreactive pyramidal cell layer of the CA1 hippocampal area appears to be preferentially vulnerable to neurotoxic actions of glucocorticoids, especially in some rat strains.
3. Strong evidence suggests that each nerve cell in the CNS is supported by a trophic unit, consisting of other nerve cells and glial cells, blood vessels, and extracellular matrix molecules. Due to multiple actions on nerve and glial cell populations of the different trophic units, the glucocorticoids may exert either an overall trophic or a neurotoxic action. It seems likely that with increasing age, the endangering actions of glucocorticoids on nerve cells prevail over the neurotrophic ones, leading to reduced nerve cell survival in some trophic units.
Similar content being viewed by others
References
Agnati, L. F., Zoli, M., Grimaldi, R., Fuxe, K., Toffano, G., and Zini, I. (1990). Cellular and synaptic alterations in the aging brain.Aging 25–25.
Agnati, L. F., Cortelli, P., Pettersson, R., and Fuxe, K. (1995). The concept of trophic units in the central nervous system.Prog. in Neurobiol. 46561–574.
Ahima, R., and Harlan, R. (1990). Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system.Neuroscience 39579–604.
Aloe, L. (1989). Adrenalectomy decreases nerve growth factor in young adult rat hippocampus.Proc. Natl. Acad. Sci. USA 865636–5640.
Alonso, J., Castellano, M. A., and Rodriguez, M. (1991). Behavioural lateralization in rats: prenatal stress effects on sex differences.Brain Res. 53945–50.
Armanini, M., Hutchins, C., Stein, B., and Sapolsky, R. (1990). Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent.Brain Res. 5327–12.
Barbany, G., and Persson, H. (1992). Regulation of neurotrophin mRNA expression in the rat brain by glucocorticoids.Eur. J. Neurobiol. 4396–403.
Barbany, G., and Persson, H. (1993). Adrenalectomy attenuates kainic acid-elicited increases of messenger RNA's for neurotrophins and their receptors in the rat brain.Neuroscience 54909–922.
Bardgett, M., Taylor, G., Csernansky, J., Newcomer, J., and Nock, B. (1994). Chronic corticosterone treatment impairs spontaneous alternation behavior in rats.Behav. Neural Biol. 61186–190.
Björklund, H., Eriksdotter-Nilsson, M., Dahl, D., Rose, G., Hoffer, B., and Olson, L. (1985). Image analysis of GFAP positive astrocytes from adolescence to senescence.Exp. Brain Res. 58163–170.
Bohn, M. C., Dean, D., Hussain, S., and Guiliano, R. (1994). Development of mRNAs for glucocorticoid and mineralocorticoid receptors in rat hippocampus.Dev. Brain Res. 77157–162.
Byron, D., Dauphin, C., and Di Paolo, T. (1992). Effects of adrenalectomy and glucocorticoids on rat brain dopamine receptors.Neuroendocrinology 55468–476.
Chadi, G., Rosen, L., Cintra, A., Tinner, B., Zoli, M., Pettersson, R., and Fuxe, K. (1993). Corticosterone increases FGF-2(bFGF) immunoreactivity in the substantia nigra of the rat.NeuroReport 4783–786.
Chadi, G., Cao, Y., Pettersson, R., and Fuxe, K. (1994). Temporal and spatial increase of astroglial basic fibroblast growth factor synthesis after 6-hydroxydopamine-induced degeneration of the nigrostriatal dopamine neurons.Neuroscience 61891–910.
Cintra, A., Solfrini, V., Agnati, L., Gustafsson, J.-Å., and Fuxe, K. (1991). Strongly glucocorticoid receptor immunoreactive neurons in the neonatal rat brain.NeuroReport 285–88.
Cintra, A., Solfrini, V., Bunneman, B., Okret, S., Bortolotti, F., Gustafsson, J.-Å., and Fuxe, K. (1993). Prenatal development of glucocorticoid receptor gene expression and immunoreactivity in the rat brain and pituitary gland: A combined in situ hybridization and immunocytochemistry analysis.Neuroendocrinology 571133–1147.
Cintra, A., Bhatnagar, M., Chadi, G., Tinner, B., Lindberg, J., Gustafsson, J.-Å., Agnati, L., and Fuxe, K. (1994a). Glial and neuronal glucocorticoid receptor immunoreactive cell populations in developing, adult, and aging brain. InBrain Corticosteroid Receptors. Studies of the Mechanism, Function, and Neurotoxicity of Corticosteroid Action (E. de Kloet, E. Azmitia, and P. Landfield, Eds.),Ann. N.Y. Sci. New York, pp. 42–63.
Cintra, A., Lindberg, J., Chadi, G., Tinner, B., Möller, A., Gustafsson, J.-Å., De Kloet, E., Oitzl, M., Agnati, L., and Fuxe, K. (1994b). Basic fibroblast growth factor and steroid receptors in the aging hippocampus of the brown Norway rat: Immunocytochemical analysis in combination with stereology.Neurochem. Int. 2539–45.
Cintra, A., Zoli, M., Rosén, L., Agnati, L., Okret, S., Wikström, A.-C., Gustafsson, J.-Å., and Fuxe, K. (1994c). Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system.Neuroscience 62843–897.
DeKloet, E. R., Sutanto, W., Rots, N., van Haarst, A., van den Berg, D., Oitzl, M., van Eekelen, J. A. M., and Voorhuis, D. (1991). Plasticity and function of brain corticosteroid receptors during aging.Acta Endocrinol. (Copenh.)125(Suppl. 1):65–72.
Deminiere, J. M., Piazza, P. V., Guegan, G., Abrous, N., Maccari, S., Le Moal, M., and Simon, H. (1992). Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers.Brain Res. 586135–139.
Diaz, R., Ögren, S. O., Blum, M., and Fuxe, K. (1995). Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor activity and brain dopamine metabolism in prepubertal male and female rats.Neuroscience 66467–473.
Eldridge, J. C., Fleenor, D. G., Kerr, D. S., and Landfield, P. W. (1989). Impaired up-regulation of type II corticosteroid receptors in hippocampus of aged rats.Brain Res. 478248–256.
Elliott, E. M., and Sapolsky, R. M. (1992). Corticosterone enhances kainic acid-induced calcium elevation in cultured hippocampal neurons.J. Neurochem. 591033–1040.
Elliott, E., Mattson, M., Vankerklish, P., Lynch, G., Chang, I., and Sapolsky, R. (1993). Corticosterone exacerbates kainate-induced tau immunoreactivity and spectrin proteolysis in vivo.J. Neurochem. 6157–67.
Ernfors, P., IbaSNOW;ez, C., Ebendal, T., Olson, L., and Persson, H. (1990). Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: Developmental and topographical expression in the brain.Proc. Natl. Acad. Sci. USA 875454–5458.
Faunt, J. E., and Crocker, A. D. (1988). Adrenocortical hormone status affects responses to dopamine receptor agonists.Eur. J. Pharmacol. 152255–261.
Fuxe, K., Wikstöm, A. C., Okret, S., Agnati, L. F., Härfstrand, A., Yu, Z.-Y., Granholm, L., Zoli, M., Vale, W., and Gustafsson, J. Å. (1985). Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor.Endocrinology 177(5):1803–1812.
Fuxe, K., Chadi, G., Agnati, L., Tinner, B., Rosén, L., Janson, A.-M., Möller, A., Cintra, A., Cao, Y., Goldstein, M., Lindahl, U., David, G., Ögren, S.-O., Toffano, G., Baird, A., and Pettersson, R. (1994). Fibroblast growth factor-2, ganglioside GM1 and the trophic regulation of the basal ganglia. Focus on the nigrostriatal dopamine neurons. InTrophic Regulation of the Basal Ganglia. Focus on Dopamine Neurons (K. Fuxe, L. Agnati, B. Bjelke, and D. Ottoson, Eds.), Elsevier, Oxford, pp. 1–41.
Gazzara, R. A., Fisher, R. S., and Howard, S. G. (1986). The ontogeny of amphetamine-induced dopamine release in the caudate-putamen of the rat.Dev. Brain Res. 28213–220.
Gilad, G. M., Rabey, J. M., and Gilad, V. H. (1987). Presynaptic effects of glucocorticoids on dopaminergic and cholinergic synaptosomes. Implications for rapid endocrine-neural interactions in stress.Life Sci. 402401–2408.
Härfstrand, A., Fuxe, K., Cintra, A., Agnati, L. F., Zini, I., Wikström, A.-C., Okret, S., Yu, Z.-Y., Goldstein, M., Steinbush, H., Verhofstad, A., and Gustafsson, J.-Å. (1986). Glucocorticoid receptor immunoreactivity in monoaminergic neurons of the rat brain.Proc. Natl. Acad. Sci. U.S.A. 839779–9783.
Horner, H., Packan, D., and Sapolsky, R. (1990). Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia.Neuroendocrinology 5257–64.
Jones, G. H., and Robbins, T. W. (1992). Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity.Pharmacol. Biochem. Behav. 43887–895.
Köhler, C., Eriksson, H., Watanabe, T., Polak, J., Palay, V., and Chan-Palay, V. (1986). Galanin immunoreactivity in hypothalamic histamine neurons: Further evidence for multiple chemical messengers in the tuberomammillary nucleus.J. Comp. Neurol. 25058–64.
Krozowski, Z., Rundle, S. E., Wallace, C., Castell, M. J., Shen, J.-H., Dowling, J., Funder, J. W., and Smith, A. I. (1989). Immunolocalization of renal mineralocorticoid receptors with an antiserum against a peptide deduced from the complementary deoxyribonucleic acid sequence.Endocrinology 125192–198.
Landfield, P., Baskin, R., and Pitler, T. (1981). Brain aging correlates: Retardation by hormonal-pharmacological treatments.Science 214581–584.
Landfield, P., Waymire, J., and Lynch, G. (1978). Hippocampal aging and adrenocorticoids: quantitative correlations.Science 2021098–1102.
Landfield, P., Sundberg, D., Smith, M., Eldridge, J., and Morris, M. (1980). Mammalian aging: Theoretical implications of changes in brain and endocrine systems during mid and late life.Peptides 1185–196.
Matsuzaki, K., Yoshitake, Y., Matuo, Y., Sasaki, H., and Nishikawa, K. (1989). Monoclonal antibodies against heparin-binding growth factor II/basic fibroblast growth factor that block its biological activity: Invalidity of the antibodies for tumor angiogenesis.Proc. Natl. Acad. Sci. USA 869911–9915.
McEwen, B. S., Cameron, H., Chao, H. M., Gould, E., Magarinos, A. M., Watanabe, Y., and Wooley, C. S. (1993). Adrenal steroids and plasticity of hippocampal neurons: Toward an understanding of underlying cellular and molecular mechanisms.Cell. Mol. Neurobiol. 13457–482.
McGeer, P. L., and Rogers, J. (1992). Anti-inflamatory agents as a therapeutic approach to Alzheimer's disease.Neurology 42447–449.
Meaney, M. J., Sapolsky, R. M., Aiteken, D. H., and McEwen, B. S. (1985).3H Dexamethosone binding in the limbic brain of the fetal rat.Dev. Brain Res. 23297–300.
Ögren, S. O., Köhler, C., Fuxe, K., and Ängeby, K. (1979). Behavioural effects of ergot drugs. InDopaminergic Ergot Derivatives and Motor Function (K. Fuxe and D. B. Calne, Eds.), Pergamon, Oxford, p. 187.
Okret, S., Wikström, A.-C., Wrange, Ö., Andersson, B., and Gustafsson, J. Å. (1984). Monoclonal antibodies against the rat liver glucocorticoid receptor.Proc. Natl. Acad. Sci. USA 811609–1613.
Poirier, J. (1994). Apolipoprotein E in animal model of CNS injury and in Alzheimer's disease.Trends Neurosci. 17525–530.
Rodriguez, M., Martin, L., and Santana, C. (1994). Ontogenic development of brain asymmetry in dopaminergic neurons.Brain Res. Bull. 33163–171.
Rosenfeld, P., van Eekelen, J. A. M., Levine, S., and de Kloet, E. R. (1993). Ontogeny of corticosteroid receptors in the brain.Cell. Mol. Neurobiol. 13295–319.
Sapolsky, R., and Pulsinelli, W. (1985). Glucocorticoids potentiate ischemic injury to neurons: Therapeutic implications.Science 2291397–1399.
Sapolsky, R. L., Krey, L. C., and McEwen, B. S. (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging.J. Neurosci. 51222–1227.
Sapolsky, R. M., Krey, L. C., and McEwen, B. S. (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis.Endocr. Rev. 7284–301.
Sapolsky, R., Stein-Behrens, B., and Armanini, M. (1991). Long-term adrenalectomy causes loss of dentate gyrus and pyramidal neurons in the adult hippocampus.Exp. Neurol. 114246.
Seckl, J. R., and Brown, R. W. (1994). 11-Beta-hydroxysteroid dehydrogenase: on several roads to hypertension.J. Hypertens. 12105–112.
Sloviter, R., Dean, E., and Neubort, S. (1993a). An electron microscopic analysis of adrenalectomy-induced hippocampal granule cell degeneration in the rat; Apoptosis in the adult central nervous system.J. Comp. Neurol. 330337–351.
Sloviter, R. S., Sollas, A. L., Dean, E., and Neubort, S. (1993b). Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus; Characterization of anin vivo model of controlled neuronal death.J. Comp. Neurol. 330324–336.
Swanson, L. W., and Simmons, D. M. (1989). Differential steroid hormone and neuronal influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: A hybridization histochemical study in the rat.J. Comp. Neurol. 285413–435.
Takahashi, L. K., Turner, J. G., and Kalin, N. H. (1992). Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats.Brain Res. 574131–137.
Tilders, F. J. H., Schmidt, E. D., and De Goeij, D. C. E. (1993). Phenotypic plasticity of CRF neurons during stress.Ann. N.Y. Acad. Sci. 69739–52.
Tombaugh, G. C., and Sapolsky, R. M. (1992a). Corticosterone accelerates hypoxia- and cyanide-induced ATP loss in cultured hippocampal astrocytes.Brain Res. 588154–158.
Tombaugh, C. G., Yang, H. S., Swanson, A. R., and Sapolsky, M. R. (1992b). Glucocorticoids exacerbate hypoxic and hyperglycemic hippocampal injury in vitro: Biochemical correlates and a role for astrocytes.J. Neurochem. 59137–145.
Uno, H., Lohmiller, L., Thieme, C., Kemnitz, J. W., Engle, M. J., Roecker, E., and Farell, P. (1990). Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus.Dev. Brain Res. 53157–167.
van Eekelen, J. A., Rots, N. Y., Sutanto, W., and de Kloet, E. R. (1992). The effect of aging on stress responsiveness and central corticosteroid receptors in the brown Norway rat.Neurobiol. Aging 13159–170.
Virgin, C. E. J., Ha, T. P.-T., Packan, D. R., Tombaugh, G. C., Yang, S. H., Horner, H. C., and Sapolsky, R. M. (1991). Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes. Implications for glucocorticoid neurotoxicity.J. Neurochem. 571422–1428.
von Euler, G., Fuxe, K., Tanganelli, S., Finnman, U.-B., and Eneroth, P. (1990). Changes in pituitary-adrenal activity affect the binding properties of striatal dopamine D2 receptors but not their modulation by neurotensin and cholecystokinin-8.Neurochem. Int. 16275–280.
Weinstock, M., Fride, E., and Hertzberg, R. (1988). Prenatal stress effects on functional development of the offspring. InProgress in Brain Research (G. J. Boer, M. G. P. Feenstra, M. Mirmiran, D. F. Swaab, and F. Van Haaren, Eds.), Elsevier Science, B.V. (Biomedical Division), Amsterdam, pp. 319–331.
Weinstock, M., Matlina, E., Maor, G. I., Rosen, H., and McEwen, B. S. (1992). Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat.Brain Res. 595195–200.
Zarrow, M. X., Philpott, J. E., and Denenberg, V. H. (1970). Passage of14C-4-corticosterone from the rat mother to the foetus and neonate.Nature 2261058–1059.
Zoli, M., Agnati, L., Fuxe, K., Ferraguti, F., Biagini, G., Cintra, A., and Gustafsson, J.-Å. (1990). Long-lasting reduction of glucocorticoid receptor immunoreactivity in the hippocampal field CA1 but not in the dentate gyrus after neonatal treatment with corticosterone in the rat.Acta Physiol. Scand. 138577–579.
Zoli, M., Ferraguti, F., Gustafsson, J.-Å., Toffano, G., Fuxe, K., and Agnati, L. F. (1991a). Selective reduction of glucocorticoid receptor immunoreactivity in the hippocampal formation and central amygdaloid nucleus of the aged rat.Brain Res. 545199–207.
Zoli, M., Ferraguti, F., Biagini, G., Cintra, A., Fuxe, K., and Agnati, L. F. (1991b). Corticosterone treatment counteracts lesions induced by neonatal treatment with monosodium glutamate in the mediobasal hypothalamus of the male rat.Neurosci. Lett. 132225–228.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fuxe, K., Diaz, R., Cintra, A. et al. On the role of glucocorticoid receptors in brain plasticity. Cell Mol Neurobiol 16, 239–258 (1996). https://doi.org/10.1007/BF02088179
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02088179