Abstract
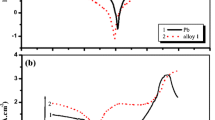
The electrochemical reactions which take place on lead and lead dioxide electrodes of a lead-acid battery are highly influenced by the presence in the electrolytic solution of ions Cd(II), Ni(II), Zn(II) and Co(II). Current density-potential curves were obtained in this work by using a Pb/Ca alloy. It was observed that Co(II) and Ni(II) ions catalyzed both hydrogen and oxygen evolution reactions. On the other hand, Cd(II) and Zn(II) ions decreased the hydrogen evolution rate.
Abstract
На электрохимические реакции, протекающие на электродах свинца и двуокиси свинца в свинцово-кислых аккумуляторах, сильно влияет присутствие в электролитическом растворе ионов Cd(II), Ni(II), Zn(II) и Co(II). Кривые плотность тока-потенциал получены, используя сплавы Pb/Ca. Найдено, что металлические ионы Co(II) и Ni(II) катализируют реакции выделения как водорода, так и кислорода. С другой стороны, металлические ионы Cd(II) и Zn(II) уменьшают скорость реакции выделения водорода.
Similar content being viewed by others
References
S. C. Barnes: Additives for Low Temperature Battery Operation, Second International Conf. on Lead. Arnhem, The Netherlands, 1965.
N. A. Hampson, J. B. Lakeman: J. Electroanal. Chem.,119, 3 (1981).
V. S. Shaldaev, K. V. Rybalka: Elektrokhimiya,15, 381 (1979).
B. K. Mahato, W. H. Tiedemann: J. Electrochem. Soc.,30, 2139 (1983).
P. Ruetschi, B. D. Cahan: J. Electrochem. Soc.,104, 406 (1957).
B. K. Mahato, J. L. Strebe, D. F. Wilkinson, K. R. Bullock: J. Electrochem. Soc.,132, 19 (1985).
H. Bode: Lead Acid Batteries. Wiley, New York, 1977.
M. Dasoyan: Dokl. Akad. Nauk SSSR,107, 863 (1956); Nauk SSSR, Khim. Tekh.
V. P. Mashovetts, A. Z. Lyandres: Zh. Prikl. Khim.,21, 441 (1947).
B. W. Burrows, W. G. Sunn, B. H. Dick: J. Energy,5, 138 (1981).
E. Willihnganz: Electrochem. Technol.,6, 338 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sánchez, H., Meas, Y. Electrocatalytic effect of metal ions on lead and lead dioxide electrode reactions. React Kinet Catal Lett 45, 67–71 (1991). https://doi.org/10.1007/BF02078610
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02078610