Abstract
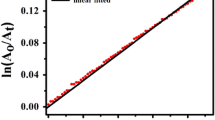
Pseudo-first order rate constants (kobs) for methanolysis of− PS− show a decrease and increase of nearly 14 and 40% with increase in MeCN content from 2 to 20 or 30 and from 20 or 30 to 60 or 70 vol.%, respectively. The increase in [KCl] from 0.0 to 3.0 mol dm−3 decreases kobs by nearly 25%.
Similar content being viewed by others
References
A. R. Fersht:Enzyme Structure and Mechanism, W.H. Freeman: San Francisco 1977;
W. P. Jencks:Catalysis in Chemistry and Enzymology, McGraw-Hill, New York 1969.
M. N. Khan, M. Yakubu:J. Chem. Res. (S), 346 (1986).
M. N. Khan, I. L. Fatope, K. I. Isaac, M. O. Zubair:J. Chem. Soc. Perkin Trans.,2, 655 (1986).
M. N. Khan, J. Naaliya, M. Dahiru:J. Chem. Res. (S), 116 (1988); (M) 1168 (1988).
M. N. Khan:Int. J. Chem. Kinet.,19, 757 (1987).
M. N. Khan, A. A. Audu:J. Phys. Org. Chem.,5, 129 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Niyaz Khan, M., Arifin, Z., Hamid Yahya, A. et al. Temperature, salt and solvent effects on intramolecular general base-catalyzed methanolysis of ionized phenyl salicylate (PS−). React Kinet Catal Lett 55, 283–289 (1995). https://doi.org/10.1007/BF02073062
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02073062