Abstract
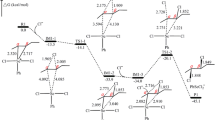
Studies of 1-ethyl-(α-14C)-indane dehydroisomerization over a Pt/Al2O3 catalyst (AP-64) have revealed that 34.5% or 16.5% of the initial tracer label migrates into the α-position of the expanded methylnaphthalene ring when the catalyst is pretreated at 763 K in Ar or H2, respectively. The reaction mechanism is discussed.
Abstract
Исследована реакция дегидроизомеризации 1-этил-(α14C)-индана на алюмоплатиновом катализаторе АП-64. Показано, что при предварительной термообработке катализатора при 763 К в токе аргона в α-положение расширенного кольца в метилнафталинах переходит 34,5% исходной метки, при обработке в токе водорода—16,5%. Обсуждается механизм реакции.
Similar content being viewed by others
References
A.G. Sakhabutdinov, A.A. Ageenko, N.G. Devyatko, V.G. Lipovich: Kinet. Katal.20, 1595 (1979).
H. Dimitrov, I. Topalova: Proc. 4th Intern. Congr. Heterogeneous Catalysis, paper 87, p. 385, Varna, 1979.
A.G. Sakhabutdinov, A.A. Ageenko, G.A. Mikhailenko, V.G. Lipovich: Izv. Vysh. Uchebn. Zaved., Khim., Khim.Tekhnol.,23, 948 (1980).
A.G. Sakhabutdinov, N.G. Devyatko, G.A. Kalabin, V.T. Lipovich: Zh. Org. Khim.,14, 311 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sergeeva, T.R., Sakhabutdinov, A.G. & Shmidt, F.K. Mechanism of ring expansion in 1-ethylindane under dehydrogenation conditions. React Kinet Catal Lett 25, 187–189 (1984). https://doi.org/10.1007/BF02064398
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02064398