Abstract
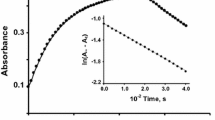
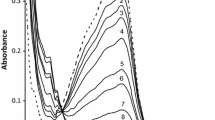
A study has been made on the oxidation of bis(2,2′,6′, 2″-terpyridine)-iron(II), Fe(tpy) 2+2 by manganese (IV) using stopped-flow spectrophotometry in H2SO4−H3PO4 mixtures. The reaction is first order in each the substrate and the oxidant. The rate of the reaction increases with hydrogen ion concentration. A plausible mechanism is proposed considering the protonated forms of manganese(IV) as reactive oxidizing species. The reaction obeys the rate law
Abstract
Проведены кинетические исследования окисления бис(2,2′,6′,2″-терпиридин)железа(II), Fe(tpy) 2+2 , марганцем(IV) в смесях H2SO4−H3PO4 с помщью спектрофотометрического метода остановленной струи. Реакция имеет первый порядок как по субстрату, так и по окислителю. Скорость реакции увеличивается с повышением концентрации иона водорода. Предложен механизм, полагающий, что протонированные формы марганца(IV) играют роль окисляющих частиц. Константа скорости реакции подчиняется следующему выражению:
Similar content being viewed by others
References
G. Crube, K. Huberich: Z. Elektrochem.,29, 8 (1923).
N. Rukmini, K. Rama Rao, V.S.N.P. Prasad: Indian J. Chem.,16A, 915 (1978).
E. Bloom, Jr., T.Y. Kometani, J.W. Mitchell, N.J. Murray: J. Inorg. Nucl. Chem.,40, 403 (1978).
N. Rukmini, V.S.N.P. Prasad, K. Rama Rao: Analyst,104, 1201 (1979).
N. Krishna Murty, G. Upendra Prasad, K. Rama Rao: Talanta,26, 1049 (1979).
T.S. Dzhabiev, V.Ya. Shafirovich, A.E. Shilov: React. Kinet. Catal. Lett.,4, 11 (1976).
V.Ya. Shafirovich, N.K. Khannanov, A.E. Shilov: React. Kinet. Catal. Lett.,19, 1502 (1978).
N.F. Tyupalo, V.V. Zamashchikov: Kinet. Katal.,24, 994 (1983).
M. Jáky, L.I. Simándi, V.Ya. Shafirovich: Inorg. Chim. Acta,90, 39 (1984).
S.K. Mandal, B.R. Sant: Talanta,23, 485 (1976).
A.A. Schilt: Analytical Applications of 1,10-Fenantroline and Related Compounds, International Series of Monographs in Analytical Chemistry, Vol. 32. Pergamon Press, New York 1976.
M.H. Ford-Smith, N. Sutin: J. Am. Chem. Soc.,83, 1830 (1961).
S.K. Mandal, B.R. Sant: J. Inorg. Nucl. Chem.,39, 2273 (1977).
K.A. Kumar, P. Sivaswaroop, K. Jaganmohan Rao, P.V. Krishna Rao: Trans. Met. Chem.,12, 441 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satyanarayana, T., Anipindi, N.R. Kinetics and mechanism of oxidation of bis(2,2′,6′,2″-terpyridine) iron(II) by manganese(IV). React Kinet Catal Lett 47, 111–118 (1992). https://doi.org/10.1007/BF02063568
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02063568