Abstract
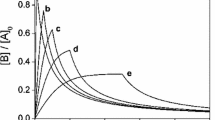
A method is proposed for the treatment of data from a kinetic system comprising two irreversible first order consecutive reactions (A→B→C). It is based on the solving of contrary propositions for an ordinary differential equation.
Abstract
Предложен метод обработки кинетических данных системы двух необратимых последовательных реакций первого порядка (A→B→C). Он основан на решении противоположных допушений обычного дифференциального уравнения.
Similar content being viewed by others
References
N.W. Alcock, D.J. Benton, P. Moore: Trans. Faraday Soc.,66, 2210 (1970).
D.W. Marquardt: J. Soc. Ind. Appl. Math.,11, 431 (1963).
W.G. Jackson, J.M. Harrowfield: Int. J. Chem. Kinet.,9, 535 (1977).
J. Casado, J.I. Gonzalez, M.N. Moreno: React. Kinet. Catal. Lett.,33, 357 (1987).
Huang Youqian: Numerical Expression and Approximation for Curve and Surface, Chapter 2. Shanghai Scientific Press, Shanghai 1981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wu, X., Zhang, W., Cai, S. et al. A method for the treatment of experimental data. Kinetic system comprising two irreversible first order consecutive reactions. React Kinet Catal Lett 47, 51–57 (1992). https://doi.org/10.1007/BF02063559
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02063559