Abstract
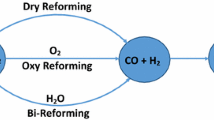
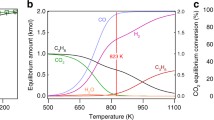
At temperatures up to 1100°C, CH4 and CO2 react over a Pt wire to give mainly the reforming product CO, even at a CH4/CO2 ratio of 4.3. But if coke is present on the wire, the dominant reaction becomes the pyrolysis of CH4 to form mainly C2H2 and C6H6. Thus, surface carbon poisons the reforming reaction and is autocatalytic for CH4 pyrolysis. Higher temperatures and larger CH4/O2 ratios favor the formation of coke and the pyrolysis reaction. Molecular oxygen and, to a lesser extent, water have the opposite effect.
Similar content being viewed by others
References
A. T. Ashcroft, A. K. Cheetham, M. L. H. Green, P. D. F. Vernon: Nature (London),352, 225 (1991).
F. Solymosi, Gy. Kutson, A. Erdőhelyi: Catal. Lett.,11, 149 (1991).
O. Takayasu, I. Matsuura, K. Nitta, Y. Yoshida: “New Frontiers in Catalysis” (L. Guczi, F. Solymosi, P. Tétényi, eds.) p. 1951–1954. Elsevier, Amsterdam, 1993.
J. Weill, F. Chevron, C. Raimbault, R. Genier, G. Renesme, L. Capogna, Y. Muller: Rev. Inst. Fr. Pet.,47, 255 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yu, Z., Choi, K., Rosynek, M.P. et al. From CH4 reforming with CO2 to pyrolysis over a platinum catalyst. React Kinet Catal Lett 51, 143–149 (1993). https://doi.org/10.1007/BF02062489
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02062489