Abstract
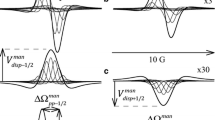
The kinetic isotope effect in proton exchange between methanol and water in CCl4 solution was studied by1H and2H NMR and kinetic IR spectroscopy. The exchange rate increases in the series CD3OH+H2O<CD3OD+H2O<CD3OD+D2O. It has been concluded that the limiting step of the reaction involves the formation of an H-bonded cyclic intermediate.
Abstract
Методами1H и2H ЯМР и кинетической ИК-спектроскопии исследован кинетический изотопний эффект в протонном обмене между метанолом и водой в растворах в CCl4. Показано, что скорость реакции увеличивается в ряду CD3OH+H2O<CD3OD+H2O<CD3OD+D2O.
Сделан вывод, что лимитирующей стадией процесса является образование цикличес-кого промежуточного комплекса с Н-связями.
Similar content being viewed by others
References
S. F. Bureiko, G. S. Denisov, R. Martsinkovskii: React. Kinet. Catal. Lett.,2, 343 (1975).
S. F. Bureiko, G. S. Denisov, K. G. Tokhadze: Studia biophysica,57, 205 (1976).
S. F. Bureiko, G. S. Denisov, I. Ya. Lange Kinet. Katal.,17, 1431 (1976).
J. Weinberg, J. R. Zimmerman: J. Chem. Phys.,23, 748 (1955).
K. C. Tewari, N. C. Li: Canad. J. Chem.,48, 1616 (1970).
S. I. Shumsky, Yu. I. Naberukhin: Zh. Strukt. Khim.,17, 182 (1976).
E. F. Caldin, S. Mateo: J. Chem. Soc. Faraday Trans. I,71, 1876 (1975).
S. F. Bureiko, G. S. Denisov: React. Kinet. Catal. Lett.,1, 283 (1974).
M. T. Rogers, J. C. Woodbrey: J. Phys. Chem.,66, 540 (1962).
S. Z. Roginskii: Teoreticheskie osnovy izotopnykh metodov izucheniya khimicheskikh reaktsii. Akad. Nauk SSSR, Moscow 1956.
I. B. Rabinovitch: in “Vodorodnaya sviaz”, p. 50. Nauka, Moscow 1964.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bureiko, S.F., Golubev, N.S., Denisov, G.S. et al. Kinetic isotope effect in proton exchange processes between methanol and water in inert solvents. React Kinet Catal Lett 7, 139–144 (1977). https://doi.org/10.1007/BF02061829
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02061829