Abstract
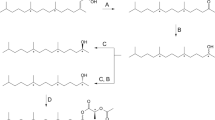
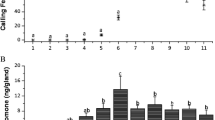
Oxime ether analogs of sex pheromone components of the turnip moth (Agrotis segetum Schiff.) were synthesized by the acidolytic opening of cyclic enol ethers withO-alkyl hydroxylamine hydrochlorides. The compounds varying in chain lengths and in the position of the C=N double bond were studied by electrophysiological single sensillum recordings (electrosen-sillography: ESG). The ESG data indicate in general reduced receptor interaction of all analogs investigated in comparison with natural pheromone components of the turnip moth. The data also show that the grade of decrease of receptor interaction depends on specific structural changes within the molecule. The results demonstrate high complementary pheromone-receptor relationships, predominantly depending on the position of the unsaturated group in the chain, whereas analogs with other structural changes are still recognized as a pheromone-like compound by the receptor.
Similar content being viewed by others

References
Arn, H., Städler, E., Rauscher, S., Buser, H.R., Mustaparta, H., Esbjerg, P., Philipsen, H., Zethner, O., Struble, D.L., andBues, R. 1980. Multicomponent sex pheromone inAgrotis segetum: Preliminary analysis and field evaluation.Z. Naturforsch. 35c:986–989.
Arn, H., Toth, M., andPriesner, E. 1992. List of sex pheromones of Lepidoptera and related attractants. Internatl. Org. Biol. Control, West Palearctic Reg. Sec. Working Group: Use of Pheromones and other Semiochemicals in Integrated Control. 180 pp.
Bengtsson, M., Liljefors, T., Hansson, B.S., Löfstedt, C., andCopaja, S.V. 1990. Structure-activity relationships for chain-shortened analogs of (Z)-5-decenyl acetate, a pheromone component of turnip moth,Agrotis segetum.J. Chem. Ecol. 16:667–684.
Bestmann, H.J. 1986. Synthesis, structure activity relationships and mode of action of insect pheromones. A contribution for the transmission of chemical information in nature, pp. 45–64,in Natural Product Chemistry. Atta-ur-Rahman (ed.). Springer-Verlag, Berlin.
Bestmann, H.J., Rösel, P., andVostrowsky, O. 1979. Alkylverzweigte Analoga von Lepitopterenpheromonen.Liebigs Ann. Chem. 1979:1189–1204.
Bestmann, H.J., Cai-Hong Wu, Döhla, B., andLi-Kedong. 1986. Functional group recognition of pheromone molecules by sensory cells ofAntherea polyphemus andAntherea pernyi (Lepidoptera: Saturniidae).Z. Naturforsch. 42c:435–441.
Camps, F., Coll, J., Fabrias, G., Guerrero, A., andRiba, M. 1984. Fluorinated analogs of insect sex pheromones.Experientia 40:933–934.
Gerecs, A., andWindholz, M. 1958. Synthesen aus Tetrahydrofurylalkohol (I).Acta Chim. Acad. Sci. Hung. 14:333–338.
Glacet, C., andGaumeton, A. 1955. The preparation and properties of 2-butylaminotetrahydropyran and 2-diethylaminotetrahydropyran.Bull. Soc. Chim. Fr. 1955:224–243.
Hansson, B.S., andBaker, T.C. 1991. Differential adaption rates in a male moth's sex pheromone receptor neurons.Naturwissenschaften 78:517–520.
Jönsson, S., Liljefors, T., andHansson, B.S. 1991. Alkyl substitution in terminal chain of (Z)-5 decenyl acetate, a pheromone component of turnip moth,Agrotis segetum. Synthesis, single-sensillum recordings, and structure-activity relationships.J. Chem. Ecol. 17:103–122.
Kaissling, K.E., andThorson, J. 1980. Insect olfactory sensilla: Structural, chemical and electrical aspects of the functional organization, pp. 261–282,in D.B. Satelle, L.M. Hall, and J.G. Hildebrand (eds.). Receptors for Neurotransmitters, Horomones and Pheromones in Insects. Elsevier/North-Holland Biomedical Press, Amsterdam.
Kleinpeter, E., andBorsdorf, R. 1981.13C-NMR-Spektroskopie in der organischen Chemie. Akademie-Verlag, Berlin.
Liljefors, T., Thelin, B., van der Pers, J.N.C., andLöfstedt, C. 1985. Chain-elongated analogs of a pheromone component of the turnip moth,Agrotis segetum. A structure-activity study using molecular mechanics.J. Chem. Soc. Perkin Trans. 2:1957–1962.
Liljefors, T., Bengtsson, M., andHansson, B.S. 1987. Effects of double-bond configuration on interaction between a moth sex pheromon component and its receptor: A receptor-interaction model based on molecular mechanics.J. Chem. Ecol. 13:2023–2040.
Potekhin, A.A., andZhdanov, S.L. 1979. Ring-chain tautomerism of 2-alkylamino-substituted tetrahydropyrans and tetrahydrofurans.Chem. Heterocycl. Comp. 15:1059–1063.
Potekhin, A.A., Zhdanov, S.L., Gindin, V.A., andOglubin, K.A. 1976. Ring-chain tautomerism of substituted hydrazones (IX). Derivatives of 5-hydroxypentanal and 4-hydroxybutanal.Zh. Org. Khim. 12:2090–2094 (Russ.), (Chem. Abstr. 86:120734s).
Prestwich, G.D., Sun, W.-C., Mayer, M.S., andDickens, J.C. 1990. Perfluorinated moth pheromones. Synthesis and electrophysiological activity.J. Chem. Ecol. 16:1761–1778.
Sun, W.-C., andPrestwich, G.D. 1990. Partially fluorinated analogs of (Z)-9-dodecenyl acetate: Probes for pheromone hydrophobicity requirements.Tetrahedron Lett. 31:801–804.
Sun, W.-C., Ng, C.-S., andPrestwich, G.D. 1992. Synthesis of partially fluorinated analogues of (Z)-5-decenyl acetate: Probes for hydrophobic interaction in pheromone reception,J. Org. Chem. 57:132–137.
Zeeh, B. andMetzger, H. 1971. Methoden zur Herstellung und Umwandlung von Hydroxylaminen. pp. 1091–1279,in Methoden Org. Chem. (Houben-Weyl), 4th ed. vol. 10/1:1195.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, D., Weber, B. Oxime ether analogs of sex pheromone components of turnip moth (Agrotis segetum Schiffermüller). J Chem Ecol 20, 1063–1073 (1994). https://doi.org/10.1007/BF02059743
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02059743