Abstract
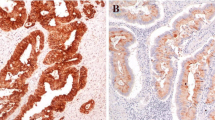
PURPOSE: The search for an understanding of the kinetics of the malignant cell is an ongoing focus of research. The aim of the present study was to determine whether there were any differences in the expression of statin, a nonproliferation-specific nuclear protein, among different colorectal tissues and whether there is any relationship between statin presence and neoplastic aggressivity. METHODS: The study population consisted of specimens from 19 patients who underwent resection for carcinoma, one for villous adenoma, and seven for colonoscopic polypectomies. Tissue samples were taken from the center of the specimen and from mucosa 10 cm from the lesion. RESULTS: Statin immunoreactivity was evaluated by counting stained nuclei in ten randomly chosen fields, and percent of positive cells was calculated. Average percentage of statin-positive cells was 34.33±6.81 in the normal crypt, 44.42±7.28 for polyps, and 7.74±5.67 for carcinomas (significantly lower than normal mucosa and polyps;P <0.001). CONCLUSION: Statin expression is dramatically diminished in invasive carcinoma tissue, but it did not help determine aggressivity with respect to Dukes stage.
Similar content being viewed by others
References
Barlogie B, Raber HN, Schumann J, Johnson TS,et al. Flow cytometry in clinical cancer research. Cancer Res 1983;43:3982–97.
Armitage NC, Robins RA, Evans DF, Turner DR, Baldwin RW, Hardcastle JD. The influence of tumor cell DNA abnormalities on survival in colorectal cancer. Br J Surg 1985;72:828–30.
Kogal W, Shribani K, Terz J, Harada JR. Tumor DNA content in the prognosis of colorectal carcinoma. JAMA 1986;255:3123–7.
Wolley RC, Schreiber K, Koss LG, Karas M, Sherman A. DNA distribution in human colon carcinomas and its relation to clinical behavior. J Natl Cancer Inst 1982;69:15–22.
Bauer KD, Lincoln ST, Vera-Roman JM,et al. Prognostic implications of proliferative activity and DNA aneuploidy in colonic adenocarcinomas. Lab Invest 1987;57:329–35.
Grinnel RS. The grading and prognosis of carcinoma of the colon and rectum. Ann Surg 1939;109:500–33.
Qualhefin RE, Gall EA. Is histologic grading of colon carcinoma a valid procedure. Arch Pathol 1953;56:466–72.
Caplejohn RS, Bone G, Awerne W. Cell proliferation in rectal carcinoma and rectal mucosa: a stathmo kinetic study. Eur J Cancer 1973;9:577–81.
Kanemitsu T, Koike A, Yamamoto S. Study of the cell proliferation kinetics in ulcerative colitis, adenomatous polyps, and cancer. Cancer 1985;56:1094–8.
Bleiberg H, Buyse M, Galand P. Cell kinetic indicators of premalignant stages of colorectal cancer. Cancer 1985;56:124–9.
Johnston PG, O'Brien MJ, Dervan PA, Carney DN. Immunohistochemical analysis of cell kinetic parameters in colonic adenocarcinomas, adenomas, and normal mucosa. Hum Pathol 1989;20:696–700.
Wang E. A 57,000-molecular weight protein uniquely present in nonproliferating cell and senescent human fibroblasts. J Cell Biol 1985;100:545–51.
Wang E. Statin, a non-proliferative-specific protein, is associated with the nuclear envelope and is heterogenously distributed in cells bearing quiescent state. J Cell Physiol 1989;140:418–26.
Wang E, Krueger JG. Application of a unique monoclonal antibody as a marker for proliferating subpopulations of cells of some tissue. J Histochem Cytochem 1985;33:587–94.
Kyzer S, Mitmaker B, Gordon PH, Schipper HM, Wang E. Proliferative activity of colonic mucosa at different distances from primary adenocarcinoma as determined by the presence of statin: a nonproliferation-specific nuclear protein. Dis Colon Rectum 1992;35:879–83.
Mitmaker B, Kyzer S, Gordon PH, Wang E. Histochemical locallization of statin—a non-proliferation-specific marker protein—in nuclei of normal and abnormal human thyroid tissue. Eur J Histochem 1992;36:123–36.
Kyzer S, Mitmaker B, Gordon PH, Schipper HM, Wang E. Rumenectomy-induced proliferation in duodenal villous epithelium is mechanistically related to the disappearance of statin, a non-proliferation-specific nuclear protein. J Histochem Cytochem 1991;39:1611–6.
Kyzer S, Gordon PH, Mitmaker B, Wang E. Proliferative activity at colonic anastomoses as determined by statin: a non-proliferation-specific nuclear protein. Dis Colon Rectum 1994;37:540–5.
Sester U, Moutsatsos IK, Wang E. A rat liver 57-kDa protein is identified to star antigenic determinants with statin, a marker for non-proliferation cells. Exp Cell Res 1989;182:550–8.
Hsu SM, Raine L, Fanger H. The use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–80.
Bogden A, Goboreanu M. Techniques of normal and pathological histochemistry. Bucharest: CERES Publishing, 1976:78–9.
Lipkin M. Phase 1 and phase 2 proliferative lesions of colonic epithelial cells in diseases leading to colonic cancer. Cancer 1974;34:878–88.
Ponz de Leon M, Roncucci L, Di Donate P,et al. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res 1988;48:2121–6.
Rew DA, Wilson GD, Taylor I, Weaver PC. Proliferation characteristics of human colorectal carcinomas measuredin vivo. Br J Surg 1991;78:60–6.
Risio M, Coverlizza S, Ferrari A, Candelaresi GL, Rossini FP. Immunohistochemical study of epithelial cell proliferation in hyperplastic polyps, adenomas, and adenocarcinomas of the large bowel. Gastroenterology 1988;94:899–906.
Terz JJ, Curutchet P, Lawrence W. Analysis of the cell kinetics of human solid tumors. Cancer 1971;5:1100–10.
Tsanaclis AM, Brem SS, Gately S, Schipper HM, Wang E. Statin immunolocalization in human brain tumors: detection of noncycling cells using a novel marker of cell quescence. Cancer 1991;68:786–92.
Trudel MA, Oligny L, Caplan S, Caplan C, Schipper HM, Wang E. Statin, a novel marker of nonproliferation: expression in nonneoplastic lymphoid tissues and follicular lymphomas. Am J Clin Pathol 1994;101:421–5.
Author information
Authors and Affiliations
Additional information
Supported by an operating grant (RO1 AGO7444) from the National Institute on Aging of the National Institutes of Health (to E.W.) and grants from the Foundation of the Sir Mortimer B. Davis-Jewish General Hospital (to P.H.G.) and the American Physician Fellowship (to S.K.).
About this article
Cite this article
Kyzer, S.D., Gordon, P.H. & Wang, E. Immunohistochemical analysis of statin in colorectal adenocarcinoma, polyps, and normal mucosa. Dis Colon Rectum 39, 546–551 (1996). https://doi.org/10.1007/BF02058709
Issue Date:
DOI: https://doi.org/10.1007/BF02058709