Abstract
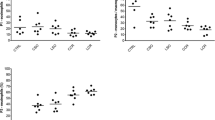
The intestinal sling procedure has been used successfully without the observance of pelvic infections. This procedure involves the implantation of polyglycolic acid (PGA) mesh to hold the bowel out of the pelvis to prevent radiation enteritis. We previously showed that PGA mesh has no intrinsic bactericidal activity. Since phagocytic leukocytes produce reactive oxygen intermediates during respiratory burst that are associated with oxygen-dependent bactericidal activity, we examined peritoneal cell types and their respiratory burst activity isolated from patients with biopsy-confirmed rectal carcinoma who underwent the intestinal sling procedure (N=12) compared with patients who did not (N=13). There was no significant difference in the cell types within the peritoneal cavity over the 7-day postoperative period examined. However, there was a significant increase in the ability of leukocytes isolated from mesh patients to produce hydrogen peroxide in the absence of an exogenous stimulus (P <0.05),as measured by flow cytometric quantitation of oxidation of the hydroperoxide-sensitive dye, 2′,7′-dichlorofluorescin diacetate (DCFH-DA). Despite the higher endogenous DCFH oxidation by leukocytes from mesh patients, the cells retained the ability to oxidize DCFH following treatment with a membrane-active stimulant for respiratory burst activity, 12-O-tetradecanoylphorbol-13-acetate. These observations suggest that PGA mesh used for the intestinal sling procedure stimulates the respiratory burst activity of peritoneal leukocytes during the postoperative period in which bacterial proliferation and colonization occur. The stimulation of reactive oxygen intermediates involved in oxygen-dependent bactericidal activity by PGA mesh may be one of the mechanisms underlying the lack of infections observed with the use of PGA mesh in contaminated settings.
Similar content being viewed by others
References
Devereux DF. The PGA sling procedure for prevention of radiation damage to the small bowel. In: Schrock TR, ed. Perspectives in colon and rectal surgery. St. Louis: Quality Medical Publishing, 1988:68–81.
Devereux DF, Chandler JJ, Eisenstat T, Zinken L. Efficacy of an absorbable mesh in keeping the small bowel out of the human pelvis following surgery. Dis Colon Rectum 1988;31:17–21.
Devereux DF, O'Connell SM, Liesch JB, Weinstein MP, Robertson FM. Induction of leukocyte activation by meshes surgically implanted in the peritoneal cavity. Am J Surg (in press).
Devereux DF, Robertson FM, Spain DA. Peritoneal leukocyte response following placement of polyglycolic acid intestinal sling in patients with rectal carcinoma (abstr). Dis Colon Rectum 1990;33:P11.
Wetter LA, Dinneen M, Levitt M, Motson RW. Wound infection after appendicectomy: results of a randomised controlled trial of dexonversus catgut and nylon wound closure (abstr). Dis Colon Rectum 1990;33:P26.
Keston AS, Brandt R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem 1965;11:1–5.
Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 1983;130:1910–7.
Hassan NF, Campbell DE, Douglas SD. Phorbol myristate acetate induced oxidation of 2′,7′-dichlorofluorescin by neutrophils from patients with chronic granulomatous disease. J Leukocyte Biol 1988;43:317–22.
Robinson PJ, Bruner LH, Bassoe C-F, Hudson JL, Ward PA, Phan SH. Measurement of intracellular fluorescence of human monocytes relative to oxidative metabolism. J Leukocyte Biol 1988;43:304–10.
Repine JE, White JG, Clawson CC, Holmes BM. The influence of phorbol myristate acetate on oxygen consumption of polymorphonuclear leukocytes. J Lab Clin Med 1978;83:911–20.
DeChatelet LR, Shirley PS, Johnston RB Jr. Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood 1976;47:545–54.
Babior BM. Oxidants from phagocytes: agents of defense and destruction, Blood 1984;64:959–66.
Klebanoff SJ. Phagocytic cells: products of oxygen metabolism. In: Gallin JI, Goldstein IM, Snyderman R, eds. Inflammation: basic principles and clinical correlates. New York: Raven Press, 1988:391.
Nathan C, Yoshida R. Cytokines: interferon-γ. In: Gallin JI, Goldstein IM, Snyderman R, eds. Inflammation: basic principles and clinical correlates. New York: Raven Press, 1988:229.
Nathan CF. Neutrophil activation on biological surfaces: massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest 1987;80:1550–60.
Klock JC, Stossel TP. Detection, pathogenesis and prevention of damage to human granulocytes caused by interaction with nylon wool fiber. J Clin Invest 1977;60:1183–90.
Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection: evidence for a local granulocyte defect. J Clin Invest 1984;73:1191–200.
Marchant RE, Miller KM, Anderson JM.In vivo biocompatibility studies. V.in vivo leukocyte interactions with biomer. J Biomed Mater Res 1984;18:1169–88.
Behling CA, Spector M. Quantitative characterization of cells at the interface of long-term implants of selected polymers. J Biomed Mater Res 1986;20:653–66.
Krause TJ, Robertson FM, Liesch JB, Wasserman AJ, Greco RS. Differential production of interleukin-1 on the surface of biomaterials. Arch Surg 1990;125:1158–60.
Liesch JB, Greco RS, Robertson FM. Characterization of leukocytes adhered to foreign body implants. J Leukocyte Biol 1987;42:412.
Robertson FM, Laskin DL, Liesch JB, Greco RS. Leukocyte activation in host-foreign body responses. J Leukocyte Biol 1987;42:414.
Author information
Authors and Affiliations
Additional information
Read in part at the meeting of The American Society of Colon and Rectal Surgeons, St. Louis, Missouri, April 29 to May 4, 1990.
About this article
Cite this article
Devereux, D.F., O'Connell, S.M., Spain, D.A. et al. Peritoneal leukocyte response following placement of polyglycolic acid intestinal sling in patients with rectal carcinoma. Dis Colon Rectum 34, 670–674 (1991). https://doi.org/10.1007/BF02050348
Issue Date:
DOI: https://doi.org/10.1007/BF02050348