Abstract
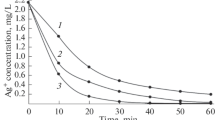
The sorption of hafnium on hydrous titanium oxide (TiO2·1.94 H2O) has been studied in detail. Maximum sorption of hafnium can be achieved from a pH 7 buffer solution containing boric acid and sodium hydroxide using 50 mg of the oxide after 30 minutes shaking. The value ofk d, the rate constant of intraparticle transport for hafnium sorption, from 0.01M hydrochloric and perchloric acid and pH 7 buffer solutions has been found to be 17 mmole·g−1·min−2. The kinetics of hafnium sorption follows Lagergren equation in 0.01M HCl solution only. The values of the overall rate constantK′=6.33·10−2 min−1 and of the rate constant for sorptionk 1=6.32·10−2 min−1 and desorptionk 2=2.28·10−5 min−1 have been evaluated using linear regression analysis. The value of correlation factor(γ) is 0.9824. The influence of hafnium concentration on its sorption has been examined from 4.55·10−5 to 9.01·10−4 M from pH 7 buffer solution. The sorption data followed only the Langmuir sorption isotherm. The saturation capacity of 9.52 mmole·g−1 and of a constant related to sorption energy have been estimated to be 2917 dm3·mole−1. Among all the additional anions and cations tested only citrate ions reduce the sorption significantly. Under optimal experimental conditions selected for hafnium sorption, As(III), Sn(V), Co(II), Se(IV) and Eu(III) have shown higher sorption whereas Mn(II), Ag(I) and Sc(III) are sorbed to a lesser extent. It can be concluded that a titanium oxide bed can be used for the preconcentration and removal of hafnium and other metal ions showing higher sorption from their very dilute solutions. The oxide can also be employed for the decontamination of radioactive liquid waste and for pollution abatement studies.
Similar content being viewed by others
References
M. Grayson, Kirk-Othmer, Encyclopedia of Chemical Technology. Vol. 12, John Wiley & Sons Inc., 3rd ed., New York, 1980, p. 73.
C. A. Hampel, G. G. Hawley, The Encyclopedia of Chemistry, Van Nostrand Reinhold, 3rd ed., New York, 1973, p. 515.
S. M. Hasany, F. A. Chughtai, A. Ghaffar, Sep. Sci. Technol., 26 (1991) 1131.
S. M. Hasany, A. M. Shamsi, M. A. Rauf, unpublished results.
S. M. Hasany, M. H. Chaudhary, J. Radioanal. Nucl. Chem., 111 (1987) 199.
S. M. Hasany, E. Haque, S. B. Butt, Sep. Sci. Technol., 21 (1986) 1125.
S.M. Hasany, M. H. Chaudhary, Sep. Sci. Technol., 21 (1986) 97.
W. J. Weber, S. C. Morris, J. San. Eng. Div. ASEC 899 (SA2) (1963) 31.
S. Lagergren, B. K. Sven, Vatens Kapsakad. Handl. (1898) 24.
D. Reichenburg, J. Am. Chem. Soc., 75 (1953) 589.
E. M. Mikhail, N. Z. Misak, J. Chem. Tech. Biotechnol. 39 (1987) 219.
L. Maya, Radiochim. Acta, 31 (1982) 147.
A. E. Martell, L. G. Sillen, Stability Constants of Metal Ion Complexes. Spec. Publ. No. 25, The Chemical Society London, 1971.
J. Kragten, Atlas of Metal Ligand Equilibrium in Aqueous Solutions, Ellis Harwood, Chichester, 1978.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasany, S.M., Shamsi, A.M. & Rauf, M.A. Sorption of hafnium on hydrous titanium oxide using radiotracer technique. J Radioanal Nucl Chem 219, 51–54 (1997). https://doi.org/10.1007/BF02040264
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02040264