Abstract
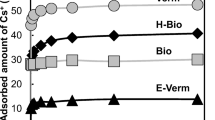
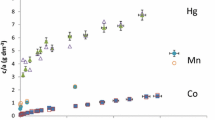
The sorption of cesium and cobalt on kaolinite and montmorillonite were followed by radiotracer method. The sorption of cesium can be described by a Freundlich isotherm. Cobalt sorption on clays equilibrated in cesium chloride solutions significantly differs for kaolinite and montmorillonite due to their differences in sites available for cation sorption and changes in solution chemistry.
Heterogeneous exchange of cesium ions between clay and the surrounding electrolyte was performed in order to obtain information about clay/electrolyte systems equilibration. An interplay of different processes included in the attainment of true chemical equilibria of clay/electrolyte systems are responsible for the change of clay surface properties and total exchange capacity.
Similar content being viewed by others
References
D. W. OSCARSON, R. L. WATSON, H. G. MILLER, Applied Clay Science, 2 (1987) 29.
I. G. McKINLEY, J. M. WEST, Rad. Waste Manag. Nucl. Fuel Cycle, 4 (1984) 379.
D. W. OSCARSON, S. C. H. CHEUNG, Evaluation of Phyllosilicates as a Buffer Component in the Disposal of Nuclear Fuel Waste, Report, AECL-7812, 1983.
A. S. BUCHANAN, R. C. OPPENHEIM, Aust. J. Chem., 21 (1968) 2367.
A. S. BUCHANAN, R. C. OPPENHEIM, Aust. J. Chem., 25 (1972) 1857.
H. Van OLPHEN, Clay Colloid Chemistry, John Wiley Sons, 1976.
H. S. HANNA, P. SOMASUNDARAN, J. Colloid Interface Sci., 70 (1974) 181.
S. LEE SCHWARTZEN-ALLEN, E. MATIJEVIĆ, Chem. Rev., 74 (1974) 385.
E. S. BUSENBERG, C. V. CLEMENCY, Clays and Clay Minerals 21 (1973) 221.
N. FILIPOVIĆ-VINCEKOVIĆ, R. DESPOTOVIĆ, Radiochim. Acta, 27 (1980) 213.
B. L. SAWHNEY, Clays and Clay Minerals, 18 (1970) 47.
B. L. SAWHNEY, Clays and Clay Minerals, 20 (1972) 93.
N. T. COLEMAN, D. CRAIG, R. J. LEWIS, Soil Sci. Soc. Amer. Proc., 27 (1963) 287.
N. T. COLEMAN, R. J. LEWIS, D. CRAIG, Soil Sci. Soc. Amer. Proc., 27 (1963) 290.
K. E. ZIEMENS, Ark. Kemi. Minerl. Geol., 20 A (1945) No. 18, 1.
R. H. H. WOLF, M. MIRNIK, B. TEŽAK, Kolloid Z. Z. Polymere, 205 (1965) 111.
J. F. HODGSON, Soil Sci. Soc. Am. Proc., 24 (1960) 165.
K. G. TILLER, J. F. HODGSON, M. PEECH, Soil Sci., 95 (1965) 392.
H. Van OLPHEN, J. Colloid Sci., 19 (1964) 313.
R. K. ILER, Colloid Chemistry of Silica and Silicates, Cornell Univ. Press, Ithaca, New York, 1955, p. 197.
E. MATIJEVIĆ, K. G. MATHAI, R. H. OTTEWILL, M. KERKER, J. Phys. Chem., 65 (1961) 826.
P. STROHAL, K. MOLNAR, I. BAČIĆ, Mikrochim. Acta, 5 (1972) 586.
E. A. JENNE, Trace Inorganics in Water, Advances in Chemistry Series 73, Amer. Chem. Soc. Washington, 1968, p. 337.
K. G. TILLER, J. F. HODGSON, Proc. 9th National Conference on Clays and Clay Minerals, Pergamon Press, New York, 1962.
D. H. SALOMON, B. C. LOFT, J. D. SWIFT, Clay Minerals, 7 (1968) 389.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Filipović-Vinceković, N., Brečević, L. & Kralj, D. Interactions in clay/electrolyte systems. Journal of Radioanalytical and Nuclear Chemistry, Articles 130, 155–167 (1989). https://doi.org/10.1007/BF02037709
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02037709