Abstract
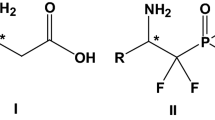
A method has been developed to convert disparlure (2-methyl-7,8-epoxyoctadecane) to the correspondingN-(α-methylbenzyl)aziridine with inversion of configuration at both the 7 and 8 positions. The diastereomeric aziridines can be separated on an efficient gas chromatography column, permitting determination of the enantiomeric constitution of the starting disparlure. As little as ca. 0.1% of the minor enantiomer can be detected.
Similar content being viewed by others
References
Arjona, O., Mardomingo, C.L., Perez-Ossorio, R., andPlumet, Y.J. 1984. Mutarrotacion e isomerizacion de iminas. XIII. EquilibrioE-Z enN-alquil-1-arilalcaniminas.Anal. Quim. 1984: 80304–80307.
Ault, A. 1973R(+)-andS(−)-phenylethylamine, pp. 932–936,in H.E. Baumgarten (ed.). Organic Synthesis, Collective Volume V. in John Wiley and Sons, New York.
Cardé, R.T., Coane, C.C., Baker, T.C., Iwaki, S., andMarumo, S. 1977. Attractancy of optically active pheromone for male gypsy moths.Environ. Entomol. 6:768–772.
Chini, M., Crotti, P., andMacchia, F. 1990. Metal salts as new catalysts for mild and efficient aminolysis of oxiranes.Tetrahedron Lett. 31:4661–4664.
Ittah, Y., Shahak, Y., Tsaroom, S., andBlum, J. 1978. A new aziridine synthesis from 2-azido alcohols and tertiary phosphines. Preparation of phenanthrene 9,10-imine.J. Org. Chem. 43:4271–4273.
Martinelli, M.J., Leanna, M.R., Varie, D.L., Peterson, B.C., Kress, T.J., Wepsiec, J.P., andKhau, V.V. 1990. The synthesis of (+)-and (-)-1-benzoyl-1,2,2a,3,4,5-hexahydrobenz[ed]indol-4-amine, and preparation of LY228729.Tetrahedron Lett. 31:7579–7582.
Miller, J.R. Mori, K., andRoelofs, W.L. 1977. Gypsy moth field trapping and electroantennogram studies with pheromone enantiomers.J. Insect Physiol. 23:1447–1453.
Mori, K. 1992. pages 85–94,in J. Apsimon, (ed.). The Total Synthesis of Natural Products, Vol. 9. John Wiley & Sons, New York.
Odell, T.M., Xu, C.-H., Schaefer, P.W., Leonhardt, B.A., Yao, D.-F. andWu, X.-D. 1992. Capture of gypsy moth,Lymantria dispar (L.), andLymantria mathura (L.) males in traps baited with disparlure enantiomers and olefin precursor in the People's Republic of China.J. Chem. Ecol. 18:2153–2159.
Overman, L.E., andFlippin, L.A. 1981. Facile aminolysis of epoxides with diethylaluminum amides.Tetrahedron Lett. 22:195–198.
Plimmer, J.R., Schwalbe, C.P., Paszek, E.C. Bierl, B.A., Webb, R.E., Marumo, S., andIwaki, S. 1977. Contrasting effectiveness of (+) and (-) enantiomers of disparlure for trapping native populations of gypsy moth in Massachusetts.Environ. Entomol. 6:518–522.
Posner, G.H. andRogers, D.Z. 1977. Organic reactions at alumina surfaces. Mild and selective opening of arene and related oxides by weak oxygen and nitrogen nucleophiles.J. Am. Chem. Soc. 99:8214–8218.
Prestwich, G.D., Graham, S.McG., andKönig, W.A. 1989. Enantioselective opening of (+)- and (-)-disparlure by epoxide hydrase in gypsy moth antennae.J. Chem. Soc. Chem. Commun. 1989:575–577.
Skidmore, M.W. 1993. Derivatives for chromatographic resolution of optically active compounds.In K. Blau and J.M. Halket (eds.). Handbook of Derivatives for Chromatography, 2nd ed. John Wiley & Sons, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oliver, J.E., Waters, R.M. Determining enantiomeric composition of disparlure. J Chem Ecol 21, 199–211 (1995). https://doi.org/10.1007/BF02036651
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02036651