Abstract
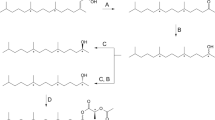
In order to test a previous conclusion that chain-elongated analogs of (Z)-5-decenyl acetate(1), a pheromone component of the turnip moth,Agrotis segetum, adopt a loop conformation of the terminal alkyl chain in the bioactive conformation, a series of alkyl ether and enol ether analogs of1 and (Z)-5-dodecenyl acetate(2) have been synthesized and tested using singlecell electrophysiology. In these analogs a methylene group in positions 7 and 9 of1 and in positions 7 and 11 in2 have been replaced by an oxygen atom in order to energetically facilitate the formation of a loop conformation in the chain-elongated analogs. The electrophysiological results in combination with molecular mechanics (MM2 and MM3) calculated conformational energies show that the activity decreases of the chain-elongated ether analogs are significantly smaller than that for2 and that these activity decreases parallel the conformational energies for a loop formation of the terminal chains in the analogs. The results support our previous conclusion that the terminal chain of chain-elongated analogs of1 adopts a loop conformation in their bioactive conformations.
Similar content being viewed by others
References
Alexakis, A., andNormant, J.F. 1984. Alkenyl copper derivatives 25: Synthesis ofE andZ enol ethers by carbocupration of alkoxy-allenes.Isr. J. Chem. 24:113–117.
Allinger, N.L., Yuh, H.Y., andLii, J.H. 1989. Molecular mechanics. The MM3 force field for hydrocarbons.J. Am. Chem. Soc. 111:8551–8566.
Bengtsson, M. 1988. Structure-activity relationships for analogues of (Z)-5-decenyl acetate, a sex pheromone component of the turnip moth,Agrotis segetum. Synthesis and conformational analysis. PhD thesis. Lund University, Sweden.
Bengtsson, M., Liljefors, T., andHansson, B.S. 1987. Dienic analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum: Synthesis, conformational analysis and structure-activity relationships.Bioorg. Chem. 15:409–422.
Bengtsson, M., Liljefors, T., Hansson, B.S., Löfstedt, C., andCopaja, S.V. 1990. Structure-activity relationship for chain-shortened analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum.J. Chem. Ecol. 16:667–684.
Burgstahler, A., Weigel, L.O., Sanders, M.E., andShaefer, C.G. 1977. Synthesis and activity of 29-hydroxy-3,11-dimethyl-2-nonancosanone, component B of the german cockroach sex pheromone.J. Org. Chem. 42:566–568.
Burkert, U., andAllinger, N. L. 1982. Molecular Mechanics. American Chemical Society, Washington D.C.
Cahiez, G., Alexakis, A., andNormant, J.F. 1978. Derives organomagnesiens ω-alcoolates: Preparation et proprietes.Tetrahedron Lett. 20:3013–3014.
Dockx, J. 1973. Quaternary ammonium compounds in organic synthesis.Synthesis 1973:441–456.
Dykyj, J., andRepas, M. 1979. The vapor pressure of organic compounds. Veda, Bratislava (in Slovak).
Gardette, M., Alexakis, A., andNormant, J.F. 1985. Carbocupration of alkynes by organocopper reagents bearing a protected hydroxy or thiol function.Tetrahedron 41:5887–5899.
Hallberg, E. 1981. Fine-structural characteristics of the antennal sensilla ofAgrotis segetum (Insecta: Lepidoptera).Cell Tissue Res. 218:209–218.
Hansch, C., andLeo, A. 1979. Substituent Constants for Correlation Analysis in Chemistry and Biology. Wiley, New York.
Hoff, S., Brandsma, L., andArens, J.F. 1968. Preparation, metallation and alkylation of allenyl ethers.Rec. Trav. Chim. 87:916–924.
Hoskovec, M., Kalinova, B., Konecn'y, K., Koutek, B., andVrkoc, J. 1993. Structure-activity correlations among analogs of the currant clearwing moth pheromone.J. Chem. Ecol. 19:735–750.
House, H.O., Chu, C.Y., Wilkins, J.M., andUmen, M.J. 1975. The chemistry of carbanions. XXVII. A convenient precursor for the generation of lithium organocuprates.J. Org. Chem. 40:1460–1469.
Houx, N.W.H., Voerman, S., andJongen, W.M.F. 1974. Purification and analysis of synthetic insect sex attractants by liquid chromatography on a silver-loaded resin.J. Chromatogr. 96:25–32.
Jönsson, S. 1991. Studies on the complementarity between a moth sex pheromone component and its receptor. Synthesis and structure-activity relationships. PhD thesis. Lund University, Sweden.
Jönsson, S., Liljefors, T., andHansson, B.S. 1991. Alkyl substitution in terminal chain of (Z)-5-decenyl acetate, a pheromone component of turnip moth,Agrotis segetum. Synthesis, single-sensillum recordings, and structure-activity relationships.J. Chem. Ecol. 17:103–122.
Jönsson, S., Liljefors, T., andHansson, B.S. 1992. Introduction of methyl groups to acetate substituted chain of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum: Synthesis, single-sensillum recordings, and structure-activity relationships.J. Chem. Ecol. 18:637–657.
Jönsson, S., Liljefors, T., andHansson, B.S. 1993. Enantiomers of methyl substituted analogs of (Z)-5-decenyl acetate as probes for the chirality and complementarity of its receptor inAgrotis segetum: Synthesis and structure-activity relationships.J. Chem. Ecol. 19:459–484.
Kaissling, K.-E. 1974. Sensory transduction in insect olfactory receptors, pp. 243–273,in L. Jaenicke (ed.). Biochemistry of Sensory Functions. Springer-Verlag, Berlin.
Kang, G.K., Kim, W.S., andMoon, B.H. 1985. An effective method for preparation of ω-bromoalkanols from α,ω-diols.Synthesis 1985:1161–1162.
Kirrman, A., andHamaide, N. 1957. Nouvelle préparation d'oxacyclanes.Bull. Soc. Chim. Fr. 1957:789–792.
Klein, H., Eijslinga, H., Westmijze, H., Meijer, J., andVermeer, P. 1976. Formation of vinylic ethers from methoxyallene and organocopper(I) compounds.Tetrahedron Lett. 18:947–948.
Liljefors, T., Thelin, B., van der Pers, J.N., andLöfstedt, C. 1985. Chain-elongated analogs of a pheromone component of the turnip moth,Agrotis segetum. A structure-activity study using molecular mechanics.J. Chem. Soc. Perkin Trans. 2:1957–1962.
Liljefors, T., Bengtsson, M., andHansson, B.S. 1987. Effects of double-bond configuration on interaction between a moth sex pheromone component and its receptor: A receptor-interaction model based on molecular mechanics.J. Chem. Ecol. 13:2023–2040.
Löfstedt, C., van der Pers, J.N.C., Löfquist, J., Lanne, B.S., Appelgren, M., Bergström, G., andThelin, B. 1982. Sex pheromone components of the turnip moth,Agrotis segetum: Chemical identification, electrophysiological evaluation and behavioral activity.J. Chem. Ecol. 8:1305–1321.
Mikami, K., Azuma, K.I., andNakai, T. 1984. [2,3]-Wittig sigmatropic rearrangement of crotyl propargyl ether system. An emerging tool for control of acyclic stereochemistry.Tetrahedron 40:2303–2308.
van der Pers, J.N.C., andden Otter, C.J. 1978. Single-cell responses from olfactory receptors of small ermine moths (Lepidoptera: Yponomeutidae) to sex attractants.J. Insect Physiol. 24:337–343.
van der Pers, J.N.C., andLöfstedt, C. 1983. Continuous single sensillum recording as a detection method for moth pheromone components in the effluent of a gas chromatograph.Physiol. Entomol. 8:203–211.
Watson, S.C., andEastham, J.F. 1967. Colored indicators for simple direct titration of magnesium and lithium reagents.J. Organomet. Chem. 9:165–168.
Wenqi, W., Bengtsson, M., Hansson, B.S., Liljefors, T., Löfstedt, C., Prestwich, G., Sun, W.-C., andSvensson, M. 1993. Electrophysiological and behavioral responses of turnip moth males,Agrotis segetum, to fluorinated pheromone analogues.J. Chem. Ecol. 19:143–157.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gustavsson, AL., Liljefors, T. & Hansson, B.S. Alkyl ether and enol ether analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum: probing a proposed bioactive conformation for chain-elongated analogs. J Chem Ecol 21, 815–832 (1995). https://doi.org/10.1007/BF02033463
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02033463