Abstract
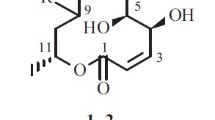
Two of the majorNuphar alkaloids, nupharolutine and 6,6′-dihydroxythiobinupharidine, were isolated from the aquatic perennial herbNuphar lutea (L.) Sibth. & Sm., yellow water lily. In a lettuce seedling bioassay of the two pure compounds, the former was inactive and the latter was highly inhibitory of radicle elongation at concentrations greater than 2 ppm. Structures and stereochemistry of the two compounds were confirmed by DEPT,1H-1H COSY,1H-13C COSY, and1H-1H NOESY experiments.
Similar content being viewed by others
References
Bohlmann, F. 1958. Zur Konfigurationsbestimmung von Chinolizidin-Derivaten.Chem. Ber. 91:2157–2167.
Cardellina, J. H., II. 1988. Natural products in the search for new agrochemicals, pp. 305–315,in H. G. Cutler, (ed.). Biologically Active Natural Products: Potential Use in Agriculture, ACS Symposium Series 380. American Chemical Society, Washington, D.C.
Cullen, W. P., LaLonde, R. T., Wang, C. J., andWong, C. F. 1973. Isolation and in vitro antifungal activity of 6,6′-dihydroxythiobinupharidine.J. Pharm. Sci. 62:826–827.
Cybulski, J. andWrobel, J. T. 1989. Nuphar alkaloids, pp. 215–257,in A. Brossi (ed.). The Alkaloids, Vol. 35, Academic Press, New York.
Duke, S. O. 1986. Microbially produced phytotoxins as herbicides—a perspective, pp. 287–304,in A. R. Putnam and C.-S. Tang (ed.). The Science of Allelopathy, John Wiley & Sons, New York.
Elakovich, S. D., andWooten, J. W. 1989. Allelopathic potential of sixteen aquatic and wetland plants.J. Aquat. Plant Manage. 27:78–84.
Elakovich, S. D., andWooten, J. W. 1991. Allelopathic potential ofNuphar lutea (L.) Sibth. & Sm. (Nymphaeaceae),J. Chem. Ecol. 17:707–714.
Elakovich, S. D., andWooten, J. W. 1995. Allelopathic, herbaceous, vascular hydrophytes, pp. 58–73,in Inderjit, K. M. M. Dakshini, and F. A. Einhellig, (eds.). Allelopathy: Organisms, Processes, and Applications, ACS Symposium Series 582. American Chemical Society, Washington, D.C.
Fischer, N. H., andQuijano, L. 1985. Allelopathic agents from common weeds, pp. 133–147,in A. C. Thompson (ed.). The Chemistry of Allelopathy: Biochemical Interactions Among Plants, ACS Symposium Series 268, American Chemical Society, Washington, D.C.
Godfrey, R. K., andWooten, J. W. 1981. Aquatic and Wetland Plants of Southeastern United States: Dicotyledons. University of Georgia Press, Athens, pp. 166–169.
Iwanow, A., Wojtasiewicz, K., andWrobel, J. 1986. Sulphoxides of thiobinupharidine thiohemiaminals fromNuphar lutea.Phytochemistry 25:2227–2231.
LaLonde, R. T., andWong, C. 1975. Thiobinupharidines epimeric at C-1 and C-1′.Can. J. Chem. 53:3545–3550.
LaLonde, R. T., andWong, C. 1977. Properties of sulfur containingNuphar alkaloids.Pure Appl. Chem. 49:169–181.
LaLonde, R. T., Wong, C. F., andCullen, W. P. 1970. Two newNuphar alkaloids: 6,6′-dihydroxythionuphlutine-A and -B.Tetrahedron Lett. 51:4477–4480.
LaLonde, R. T., Wong, C. F., andDas, K. C. 1972. A dimer alkaloid of 6,7β-oxidodeoxynupharidine.J. Am. Chem. Soc. 94:8522–8527.
LaLonde, R. T., Wong, C. F., andDas, K. C. 1974. New monohemiaminal derivatives of thiobinupharidine and thionuphlutine B. Role of circular dichroism and mass spectrometry in ascertaining the position of the hemiaminal function.J. Org. Chem. 39:2892–2897.
McClure, J. W. 1970. Secondary constituents of aquatic angiosperms, pp. 233–268,in J. B. Harborne (ed.). Phytochemical Phylogeny. Academic Press, New York.
Rice, E. R. 1984. Allelopathy, 2nd ed. Academic Press, New York.
Tatarov, A. P. 1945. White and yellow waterlilies.Farmatsiya 8:29–31,Chem. Abstr. 41:2210i (1947).
Wooten, J. W., andElakovich, S. D. 1991. Comparisons of potential allelopathy of seven freshwater species of spikerushes (Eleocharis).J. Aquat. Plant Manage. 29:12–15.
Wrobel, J. T. 1977.Nuphar alkaloids, pp. 181–214,in R. H. F. Manske (ed.). The Alkaloids. Academic Press, New York.
Wrobel, J. T., andRuszkowska, J. 1979. Chemistry of C-30 alkaloids from yellow waterlily—Nuphar luteum Sm., pp. 157–160,in J. Kovac (ed.). Collect Lectures, 3rd International Symposium Furan Chemistry. Slovak Technical University, Bratislava, Czechoslovakia.
Wrobel, J. T., Iwanow, A., Braekman-Danheux, C., Martin, T. I., andMacLean, D. B. 1972a. The structure of nupharolutine, an alkaloid ofNuphar luteum.Can. J. Chem. 50:1831–1837.
Wrobel, J. T., Iwanow, A., Szychowski, J., Poplawski, J., Yu, C. K., Martin, T. I., andMacLean, D. B. 1972b. Neothiobinupharidine sulfoxide, a new alkaloid ofNuphar luteum.Can. J. Chem. 50:1968–1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elakovich, S.D., Yang, J. Structures and allelopathic effects ofNuphar alkaloids: Nupharolutine and 6,6′-dihydroxythiobinupharidine. J Chem Ecol 22, 2209–2219 (1996). https://doi.org/10.1007/BF02029541
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02029541