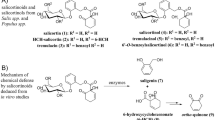
Abstract
Caterpillars ofPapilio polyxenes, the black swallowtail, feed on umbellifers that contain both toxic furanocoumarins and methylenedioxyphenyl compounds such as myristicin and safrole. These phytosynergists enhance the toxicity of furanocoumarins by inhibiting mixed-function oxidases (MFOs), the detoxification enzymes responsible for metabolizing furanocoumarins. In model substrate assays, MFOs fromP. polyxenes are twice as active as MFOs fromHeliothis zea, a generalist herbivore not adapted to feeding on either furanocoumarins or furanocoumarin/phytosynergist combinations.P. polyxenes MFOs are 10 and 46 times less sensitive to inhibition by myristicin and safrole, respectively, thanH. zea MFOs and eight times less sensitive to inhibition by safrole than MFOs fromPapilio troilus, a closely related species that does not encounter furanocoumarin/phytosynergist combinations in its diet. Higher MFO activity and decreased sensitivity to MFO inhibitors are important adaptations that allow black swallowtail caterpillars to feed on many umbelliferous plants.
Similar content being viewed by others
References
Berenbaum, M. 1981. Effects of linear furanocoumarins on an adapted specialist insect (Papilio polyxenes).Ecol. Entomol. 6:345–351.
Berenbaum, M. 1983. Coumarins and caterpillars: A case for coevolution.Evolution 37:163–179.
Berenbaum, M., andNeal, J.J. 1985. Synergism between myristicin and xanthotoxin, a naturally cooccurring plant toxicant.J. Chem. Ecol. 11:1349–1358.
Berenbaum, M., andNeal, J.J. 1986. Interactions among allelochemicals and insect resistance in crop plants, pp. 416–430,in G.R. Waller (ed.). Allelochemicals: Role in Agriculture and Forestry, American Chemical Society Symposium Series No. 330. American Chemical Society, Washington, D.C.
Brattsten, L.B., andWilkinson, C.F. 1973. Induction of microsomal enzymes in the southern armyworm (Prodenia eridania).Pest. Biochem. Physiol. 3:393–407.
Bull, D.L., Ivie, G.W., Beier, R.C., Pryor, N.W., andOertli, E.H. 1984. Fate of photosensitizing furanocoumarins in tolerant and sensitive insects.J. Chem. Ecol. 10:893–911.
Bull, D.L., Ivie, G.W., Beier, R.C., andPryor, N.W. 1986. In vitro metabolism of a linear furanocoumarin (8-methoxypsoralen, xanthotoxin) by mixed-function oxidases of larvae of the black swallowtail butterfly and the fall armyworm.J. Chem. Ecol. 12:885–892.
Crankshaw, D.L., Hetnarski, H.K., andWilkinson, C.F. 1979. Microsomal NADPH-cytochromec reductase from the midgut of the southern armyworm (Spodoptera eridania).Insect Biochem. 9:43–48.
Dixon, M. 1953. The determination of enzyme inhibitor constants.Biochem J. 55:170–171.
Gant, R.E., andClebsch, E.E.C. 1975. The allelopathic influences ofSassafras albidum in old-field succession in Tennessee.Ecology 56:604–615.
Hansen, L.G., andHodgson, E. 1971. Biochemical characteristics of insect microsomes:N- andO-Demethylation.Biochem. Pharmacol. 20:1569–1578.
Hodgson, E., andPhilpot, R.M. 1974. Interaction of methylenedioxyphenyl (1,3-benzodioxole) compounds with enzymes and their effect on mammals.Drug Metab. Rev. 3:231–301.
Ivie, G.W., Bull, D.L., Beier, R.C., Pryor, N.W., andOertli, E.H. 1983. Metabolic detoxification: mechanism of insect resistance to plant psoralens.Science 22:374–376.
Kleber, C. 1899. The chemistry of assafras.Am. J. Pharm. 71:27–32.
Krieger, R.I., Feeny, P.P., andWilkinson, C.F. 1971. Detoxification enzymes in the guts of caterpillars: An evolutionary answer to plant defenses?Science 172:579–581.
Murray, R.D.H., Mendez, J., andBrown, S.A. 1982. The Natural Coumarins. John Wiley & Sons, New York.
Neal, J.J. 1987. Ecological aspects of insect detoxication enzymes and their interaction with plant allelochemicals. Doctoral dissertation. University of Illinois at Urbana-Champaign.
Nitao, J.K. 1987. Adaptations to furanocoumarins ofPastinaca sativa byDepressaria pastinacella. Doctoral dissertation. University of Illinois at Urbana-Champaign.
Rose, H.A. 1985. The relationship between feeding specialization and host plants to aldrin epoxidase activities of midgut homogenates in larval Lepidoptera.Ecol. Entomol. 10:455–467.
Tyler, H.A. 1975. The Swallowtail Butterflies of North America. Naturegraph, Healdsburg, California.
Waldbauer, G.P., Cohen, R.W., andFriedman, S. 1984. An improved procedure of laboratory rearing of the corn earworm,Heliothis zea (Lepidoptera: Noctuidae).Great Lakes Entomol. 17:113–118.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neal, J.J., Berenbaum, M. Decreased sensitivity of mixed-function oxidases frompapilio polyxenes to inhibitors in host plants. J Chem Ecol 15, 439–446 (1989). https://doi.org/10.1007/BF02027803
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02027803