Abstract
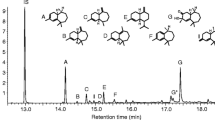
Existence of an aggregation pheromone was demonstrated inDrosophila mulleri. Mature males produce at least two compounds that are lacking from females and newly emerged males and that attract both males and females in a wind-tunnel bioassay. These compounds are (S)-(+)-2-tridecanol acetate and (Z)-10-heptadecen-2-one. Both were synthesized, and the flies responded to the synthetic compounds as well as to fly-derived preparations. The flies also responded to racemic 2-tridecanol acetate but not to the pureR enantiomer. A more polar, very volatile attractant was also extracted from both sexes ofD. mulleri but was not identified.
Similar content being viewed by others
References
Bartelt, R.J., andJackson, L.L. 1984. Hydrocarbon component of theDrosophila virilis (Diptera: Drosophilidae) aggregation pheromone: (Z)-10-heneicosene.Ann. Entomol. Soc. Am. 77:364–371.
Bartelt, R.J., Schaner, A.M., andJackson, L.L. 1986. Aggregation pheromones in five taxa of theDrosophila virilis species group.Physiol. Entomol. 11:367–376.
Bartelt, R.J., Schaner, A.M., andJackson, L.L. 1988. Aggregation pheromones inDrosophila borealis andDrosophila littoralis.J. Chem. Ecol. 14:1319–1327.
Heath, R.R., andSonnet, P.E. 1980. Technique for in situ coating of Ag+ onto silica gel in HPLC columns for the separation of geometrical isomers.J. Liq. Chromatogr. 3:1129–1135.
Miyashita, M., Yoshikoshi, A., andGrieco, P.A. 1977. Pyridiniump-toluenesulfonate: A mild and efficient catalyst for the tetrahydropyranylation of alcohols.J. Org. Chem. 42:3772–3774.
Moats, R.A., Bartelt, R.J., Jackson, L.L., andSchaner, A.M. 1987. Ester and ketone components of the aggregation pheromone ofDrosophila hydei.J. Chem. Ecol. 13:451–462.
Mori, K. 1976. Synthesis of optically active forms of ipsenol, the pheromone ofIps bark beetles.Tetrahedron 32:1101–1106.
Mori, K. 1985. Chiral synthesis: Examples in the pheromone field, pp. 293–306,in N.F. Janes (ed.). Recent Advances in the Chemistry of Insect Control. Royal Society of Chemists, London.
Pickard, R.H., andKenyon, J. 1914. Investigations on the dependence of rotatory power on chemical constitution. Part V. The simpler esters of the carbinols CH3-CH(OH)-R.J. Chem. Soc. 105:830–898.
Schaner, A.M., Bartelt, R.J., andJackson, L.L. 1987. (Z)-11-Octadecenyl acetate as an aggregation pheromone inDrosophila simulans.J. Chem. Ecol. 13:1777–1786.
Slessor, K.N., King, G.G.S., Miller, D.R., Winston, M.L., andCutforth, T.L. 1985. Determination of chirality of alcohol or latent alcohol semiochemicals in individual insects.J. Chem. Ecol. 11:1659–1667.
Suguro, T., andMori, K. 1979. Synthesis of optically active forms of 10-methylododecyl acetate, a minor component of the pheromone complex of the smaller tea tortrix moth.Agric. Biol. Chem. 43:869–870.
Yates, F. 1940. The recovery of interblock information in balanced incomplete block designs.Ann. Eugen. 10:317–325.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bartelt, R.J., Schaner, A.M. & Jackson, L.L. Aggregation pheromone components inDrosophila mulleri . J Chem Ecol 15, 399–411 (1989). https://doi.org/10.1007/BF02027800
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02027800