Summary
The calcified matrix of the hen eggshell has been demineralized with the EDTA. Aliquots of this material are soluble in water and have been characterized by column chromatography and by chemical analyses.
Of particular interest is the high hexosamine and uronic acid content, which confirms the protein-polysaccharide nature of this water-soluble material.
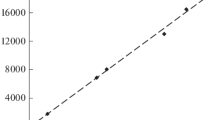
The calcium ion binding to the eggshell matrix has been studied by the equilibrium dialysis technique at different pH values, with both free and blocked carboxylic groups. The material with the free carboxylic side chain groups binds more calcium ions with increasing pH value. When the carboxylic groups have been previously blocked with a water-soluble carbodiimide, the calcium ion binding rapidly decreases. The residual capacity to bind calcium ions in the material with the carboxylic functions modified is probably due to the sulfate ions.
In agreement with previous observations on other calcified substrates, the calcium ion binding seems to depend on the presence of ionized carboxylic functions of the matrix.
Similar content being viewed by others
References
Urry, D.W., Cunningham, W.D., Ohnishi, T.: Studies on the conformation and interactions of elastin. Proton magnetic resonance of the repeating pentapeptide, Biochemistry13:609–616, 1974
Urry, D.W.: Neutral sites for calcium ion binding to elastin and collagen: A charge neutralization theory for calcification, and its relationship to atherosclerosis, Proc. Natl. Acad. Sci. U.S.A.68:810–814, 1971
Meenakshi, V.R., Donnay, G., Blackwelder, P.L., Wilbur, K.M.: The influence of substrate on calcification patterns in molluscan shell, Calcif. Tissue Res.15:31–44, 1974
Baker, J.R., Balch, D.A.: A study of the organic material of Hen's-egg shell, Biochem. J.82:352–361, 1962
Cessi, C., Piliego, F.: The determination of amino sugar in the presence of amino acids and glucose, Biochem. J.77:508, 1960
Davidson, E.A.: Modified carbazole procedure, Meth. Enzymol.8:55, 1966
Dische, Z.: Carbazole method for uronic acid, J. Biol. Chem.167:189, 1947
Scott, T.A., Melvin, E.H.: Determination of dextran with anthrone, Anal. Chem.25:1656–1661, 1953
Dodgson, K.S., Price, R.C.: A note on the determination of the ester sulphate content of sulphated polysaccharides, Biochem. J.84:106–110, 1962
Warren, L.: The thiobarbituric acid assay of sialic acids, J. Biol. Chem.234:1971, 1959
Hoare, D.G., Koshland, D.E.: A method for the quantitative modification and estimation of carboxylic acid groups in proteins, J. Biol. Chem.242:2447, 1967
Bernardi, G., Giro, M.G., Gaillard, C.: Chromatography of polypeptides and proteins on hydroxyapatite columns some new developments, Biochim. Biophys. Acta278:409–420, 1972
Bannister, D.W., Candlish, J.K., Freeman, H.: Some effects of feeding lathyrogens to laying fowls, Br. Poult. Sci.12:129–136, 1971
Candlish, J.K., Scougall, R.K.: L-5-Hydroxylysine as a constituent of the shell membranes of the hen's egg, Int. J. Protein Res.1:299–302, 1969
Cooke, A.S., Balch, D.A.: Studies of membrane, mammilary cores and cuticle of the hen egg shell, Br. Poult. Sci.11:345–352, 1970
Cooke, A.S., Balch, D.A.: The distribution and carbohydrate composition of the organic matrix in hen egg shell, Br. Poult. Sci.11:353–365, 1970
Heaney, R.K., Robinson, D.S.: The isolation and characterisation of hyaluronic acid in egg shell, Biochim. Biophys. Acts451:133–142, 1976
Krampitz, Von G., Engels, J., Heindl, I., Heinrich, A., Hamm, M., Faust, R.: Biochemische untersuchungen an eischalen, Arch. Geflügelkunde6:197–205, 1974
Abatangelo, G., Daga-Gordini, D.: Ca2+ binding study on some low molecular weight elastin peptides, Biochim. Biophys. Acta342:281–289, 1974
Abatangelo, G., Daga-Gordini, D., Garbin, G., Cortivo, R.: Calcium ion binding study on α-elastin, Biochim. Biophys. Acta371:526–533, 1974
Hauschka, P.V., Lian, J.B., Gallop, P.M.: Direct identification of the calcium binding amino acid, γ-carboxyglutamate, in mineralized tissue, Proc. Natl. Acad. Sci. U.S.A.72:3925–3929, 1975
Molinari-Tosatti, M.P., Gotte, L.: Some features of the binding of calcium ions to elastin, Calcif. Tissue Res.6:329–334, 1971
Partridge, S.M., Davis, H.F., Adair, G.S.: The chemistry of connective tissue. 2. Soluble proteins derived from partial hydrolyses of elastin, Biochem. J.61:11–12, 1955
Howard, J.B., Nelsestuen, G.L.: Properties of a Ca2+ binding peptide from prothrombin, Biochem. Biophys. Res. Commun.59:757–763, 1974
Stenflo, J., Ganrot, P.O.: Binding of Ca2+ to normal and dicoumarol-induced prothrombin, Biochem. Biophys. Res. Commun.50:98–104, 1973
Nelsestuen, G.L., Zytkovicz, T.H., Howard, J.B.: Identification of γ-carboxyglutamic acid as a component of prothrombin, J. Biol. Chem.249:6347–6350, 1974
Stenflo, J.: Structural comparison of an NH2-terminal fragment from normal and from dicoumarol-induced bovine prothrombin. J. Biol. Chem.248:6325–6332, 1973
Magnusson, S., Sottrup-Jensen, L., Peterson, T.E., Morris, H.R., Dell, A.: Primary structure of the vitamin K-dependent part of prothrombin, FEBS Lett.44:189–193, 1974
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abatangelo, G., Daga-Gordini, D., Castellani, I. et al. Some observations on the calcium ion binding to the eggshell matrix. Calc. Tis Res. 26, 247–252 (1978). https://doi.org/10.1007/BF02013266
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02013266