Summary
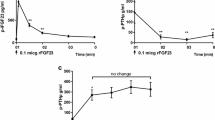
The effects of 7S nerve growth factor (NGF) and its isolated α, β, and γ subunits on bone resorption were assessed in a tissue culture system in which the degree of resorption was determined by measuring the release of45Ca from prelabeled fetal rat radii and ulnae. It was found that 7S-NGF, through the activity of its γ, subunit, inhibits parathyroid hormone (PTH)-stimulated but not-unstimulated bone resorption.
The following observations suggest that γ-NGF, a trypsin-like molecule, blocks PTH-induced bone resorption by enzymatic degradation of PTH: (a) γ-NGF does not inhibit bone resorption stimulated by the steroid, 1,25-dihydroxycholecalciferol; (b) trypsin is as effective as γ-NGF in inhibiting PTH-stimulated bone resorption; (c) the PTH-inhibitory action of both γ-NGF and trypsin are eliminated by inactivating these enzymes with diisopropyl fluorophosphate; and (d) addition of γ-NGF to the cultures 2 days after the inclusion of PTH does not result in inhibition of bone resorption. Similarly, when the subunit is added to the culture medium before the hormone, there is no inhibition of resorption. The latter observation suggests that the target of γ-NGF is the PTH molecule rather than its membrane receptors.
Crystalline bovine insulin inhibits the γ-NGF suppression of PTH-induced bone resorption. This effect, however, is not mimicked by the addition of zinc, which is present in commerical insulin preparations, to the culture medium. Consequently, insulin must inhibit NGF by some mechanism other than the influence of zinc.
Similar content being viewed by others
References
Angeletti, R.H., Hermodson, M.A., Bradshaw, R.A.: Amino acid sequences of mouse 2.5S nerve growth factor, Biochemistry12:100–115, 1973
Saide, J.D., Murphey, R.A., Canfield, R.E., Skinner, J., Robinson, D.R., Arnason, B., Young, M.: Nerve growth factor in human serum and its secretion by human cells in culture. J. Cell Biol.67:376A, 1975
Siggers, D.C., Rogers, J.G., Boyer, S.H., Margolet, L., Dorkin, H., Banerjee, S.P., Shooter, E.M.: Increased nerve-growth-factor β-chain cross-reacting material in familial dysautonomia. N. Engl. J. Med.295:629–634, 1976
Cohen, S., Levi-Montalcini, R.: A nerve growth-stimulating factor isolated from snake venom, Proc. Natl. Acad. Sci. U.S.A.42:571–574, 1956
Bocchini, V., Angeletti, P.U.: The nerve growth factor: Purification as a 30,000-molecular-weight protein, Proc. Natl. Acad. Sci. U.S.A.64:787–794, 1969
Hogue-Angeletti, R.A., Bradshaw, R.A.: Snake venoms. In C.Y. Lee (ed.): Handbook of Experimental Pharmacology. Springer-Verlag, Berlin (in press)
Bradshaw, R.A., Young, M.: Nerve growth factor. Recent developments and perspectives, Biochem. Pharmacol.25:1445–1449, 1976
Waddell, W.R., Bradshaw, R.A., Goldstein, N.M., Kirsch, W.M.: Production of human nerve-growth factor in a patient with a liposarcoma, Lancet1:1365–1367, 1968
Schenkein, I., Bueker, E.D., Helson, L., Axelrod, F., Dancis, J.: Increased nerve-growth stimulating activity in disseminated neurofibromatosis, N. Engl. J. Med.290:613–614, 1974
Bigazzi, M., Revoltella, R., Casciano, S., Vigneti, E.: High level of a nerve growth factor in the serum of a patient with medullary carcinoma of the thyroid gland, Clin. Endocrinol.6:105–112, 1977
Angeletti, R.H., Bradshaw, R.A.: Nerve growth factor from mouse submaxillary gland: Amino acid sequence, Proc. Natl. Acad. Sci. U.S.A.68:2417–2420, 1971
Varon, S., Nomura, J., Shooter, E.M.: Reversible dissociation of the mouse nerve growth factor protein into different subunits, Biochemistry7:1296–1303, 1968
Levi-Montalcini, R., Angeletti, P.U.: Immunosympathectomy, Pharmacol. Rev.18:619–628, 1966
Greene, L.A., Shooter, E.M., Varon, S.: Subunit interaction and enzymatic activity of mouse 7S nerve growth factor, Biochemistry8:3735–3741, 1969
Frazier, W.A., Boyd, L.F., Szutowicz, A., Pulliam, M.W., Bradshaw, R.A.: Specific binding sites for125I-nerve growth factor in peripheral tissues and brain, Biochem. Biophys. Res. Commun.57:1096–1103, 1974)
Bradshaw, R.A., Hogue-Angeletti, R.A., Frazier, W.A.: Nerve growth factor and insulin: Evidence of similarities in structure, function and mechanism of action. Recent Prog. Horm. Res.30:575–596, 1974
Raisz, L.F.: Bone resorption in tissue culture. Factors influencing the response to parathyroid hormone, J. Clin. Invest.44:103–116, 1965
Jeng, I.M., Bradshaw, R.A.: The preparation of nerve growth factor. In N. Marks, and R. Rodnight (eds.). Research Methods in Neurochemistry. Plenum Publishing Co., New York, pp. 265–288, 1978.
Silverman, R.E.: Interactions within the mouse nerve growth factor complex. Ph.D. thesis, Washington University, St. Louis, 1977
Greene, L.A., Shooter, E.M., Varon, S.: Enzymatic activities of mouse nerve growth factor and its subunits, Proc. Natl. Acad. Sci. U.S.A.60:1383–1388, 1968
Hartley, B.S.: Proteolytic enzymes, Annu. Rev. Biochem.29:45–72, 1960
Brewer, H.B., Fairwell, T., Rittel, W., Littledike, T., Arnaud, C.: Recent studies on the chemistry of human, bovine and procine parathyroid hormones, Am. J. Med.56:759–766, 1974
Tregear, G.W., Van Rietschoten, J., Greene, E., Keutmann, H.T., Niall, H.D., Reit, B., Parsons, J.A., Potts, J.T.: Bovine parathyroid hormone: Minimum chain length of synthetic peptide required for biological activity, Endocrinology93:1349–1353, 1973
Silverman, R., Yalow, R.S.: Heterogeneity of parathyroid hormone. Clinical and physiologic implications, J. Clin. Invest.52:1958–1971, 1973
Segre, G.V., Niall, H.D., Habener, J.F., Potts, J.T.: Metabolism of parathyroid hormone: Physiologic and clinical significance, Am. J. Med.56:774–784, 1974
Martin, K., Hruska, K., Greenwalt, A., Klahr, S., Slatopolsky, E.: Selective uptake of intact parathyroid hormone by the liver: Differences between hepatic and renal uptake, J. Clin. Invest.58:781–788, 1976
Bocci, V.: Catabolism of plasma proteins. In A.C. Allison (ed.): Structure and Function of Plasma Proteins. Vol. 2, pp. 163–188. Plenum Press, New York 1976.
Pattison, W.E., Dunn, M.F.: On the mechanism of divalent metal ion chelator induced activation of the 7S nerve growth factor esteropeptidase. Thermodynamics and kinetics of activation, Biochemistry14:2733–2739, 1975
Kaplan, E.L., Tager, H.S., Lagocki, R.: Calcium elevating peptide from pig pancreas, Clin. Res.25:622A, 1977
Young, M., Saide, J.D., Murphey, R.A., Arnason, B.G.M.: Molecular size of nerve growth factor in dilute solution, J. Biol. Chem.251:459–464, 1976
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teitelbaum, S.L., Andres, R.Y., Cooke, N.E. et al. Inhibition of parathyroid hormone-induced fetal rat bone resorption in vitro by nerve growth factor. Calc. Tis Res. 26, 203–208 (1978). https://doi.org/10.1007/BF02013259
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02013259