Abstract
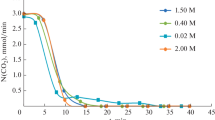
Surfaces of tooth enamel were interfaced with recirculating solutions containing calcium and phosphate. The kinetic interfacial reactions of calcium, phosphate, fluoride, and hydrogen ions were determined in solutions of constant fluoride ion concentrations (0.012, 0.025, 0.050, 0.250, or 0.500 mM/l) and /or constant pH (6.8, 7.0, 7.2, or 7.4). The rates of the calcium, phosphate, fluoride, and hydrogen ion reactions at the solid-solution interface increased under the following conditions: (1) increase of the enamel surface area; (2) increase of the rates of solution flow to the interface; (3) increase of solution pH; and (4) increase of the solution fluoride ion concentration. The nearly proportional rates of the concurrently measured reactions indicated the formation of apatitic minerals on the enamel surfaces throughout the time of solid-solution interface.
Résumé
Les surfaces d'émail dentaire sont confrontées avec des solutions de calcium et phosphate, qui y recirculent. Les réactions cinétiques au niveau des surfaces en calcium, phosphate, fluorure et ions hydrogène sont déterminées dans des solutions à concentrations en ions fluor constantes (0.012, 0.025, 0.050, 0.250 ou 0.500 mM/l) et/ou à pH constant (6.8, 7.0, 7.2 ou 7.4). Les vitesses de réactions des ions calcium, phosphate, hydrogène et fluorures augmentent dans les conditions suivantes: 1) augmentation de la surface d'émail, 2) augmentation des vitesses d'écoulement au niveau des surfaces, 3) augmentation du pH de la solution et 4) augmentation de la concentration en ion fluorure. Les vitesses presque proportionnelles des réactions mesurées simultanément indiquent la formation de minéraux apatitiques à la surface de l'émail pendant tout le temps de contact solide-solution.
Zusammenfassung
Oberflächen von Zahnschmelz wurden mit konstant zirkulierenden Lösungen, welche Calcium und Phosphate enthielten, equilibriert. Die kinetischen Oberflächenreaktionen von Calcium-, Phosphat-, Fluori- und Wasserstoff-Ionen wurden in Lösungen mit konstanten Fluoridkonzentrationen (0,012, 0,025, 0,050, 0,250 oder 0,500 mM/l) und/oder konstantem pH (6,8, 7,0, 7,2 oder 7,4) bestimmt. Die Reaktionsgeschwindigkeiten dieser Ionen an der Grenze zwischen fester und flüssiger Phase erhöhten sich unter folgenden Bedingungen: 1. Vergrößerung der Schmelzoberfläche; 2. Erhöhung der Geschwindigkeit der der Oberfläche zugeführten Lösungen; 3. Erhöhung des pH der Lösung; 4. Erhöhung der Fluoridionenkonzentration in der Lösung. Die beinahe proportionalen Geschwindigkeiten der gleichzeitig gemessenen Reaktionen deuteten darauf hin, daß auf den Schmelzoberflächen während der ganzen Equilibrierungszeit Apatitminerale gebildet wurden.
Similar content being viewed by others
References
Brown, W. E.: Crystal growth of bone mineral. Clin. Orthop.44, 205–220 (1966).
Chen, P. S., Toribara, T. Y., Warner, H.: Microdetermination of phosphorus. Analyt. Chem.28, 1756–1758 (1956).
Elliott, J. C.: Recent progress in the chemistry, crystal chemistry and structure of the apatites. Calc. Tiss. Res.3, 293–307 (1969).
Feagin, F., Koulourides, T., Pigman, W.: The characterization of enamel surface demineralization, remineralization and associated hardness changes in human and bovine material. Arch. oral Biol.14, 1407–1417 (1969).
Feagin, F., Patel, P. R., Koulourides, T., Pigman, W.: Study of the effect of calcium, phosphate, fluoride and hydrogen ion concentrations on the remineralization of partially demineralized human and bovine enamel surfaces. Arch. oral Biol.16, 535–548 (1971).
Francis, M. D., Gray, J. S., Griebstein, W. J.: The formation and influence of surface phases on calcium phosphate solids. Arch. oral. Biol.3, 83–120 (1968).
Head, J. H.: A study of saliva and its action on tooth enamel in reference to its hardening and softening. J. Amer. med. Ass.59, 2118–2122 (1912).
Johansson, B.: Remineralization of slightly etched mineral. J. dent. Res.44, 64–70 (1965).
Koulourides, T.: Remineralization of enamel and dentin, in Dental Clinics of North America, p. 485–497. Philadelphia: W. B. Saunders 1962.
Koulourides, T., Cueto, H., Pigman, W.: Rehardening of softened enamel surfaces of human teeth by solutions of calcium phosphate. Nature (Lond.)189, 226–227 (1961).
Light, T. S.: Ion-selective electrodes: Proceedings of the Symposium, Jan 30–31, 1969, R. A. Durst, ed., p. 314. Washington, D.C.: National Bureau of Standards 1969.
McCann, H. G.: The solubility of fluorapatite and its relationship to that of calcium fluoride. Arch. oral Biol.13, 987–1001 (1968).
McCann, H. G.: Reactions of fluoride ion with hydroxyapatite. J. biol. Chem.201, 247–259 (1953).
McConnell, D.: Crystal chemistry of hydroxyapatite, its relation to bone mineral. Arch. oral Biol.10, 421–431 (1965).
Miller, W. D.: The presence of bacterial plaques on the surface of teeth and their significance. Dent. Cosmos44 (5), 425–446 (1902).
Muhlemann, H. R., Lenz, H., Rossinsky, K.: Electron microscopic appearance of rehardened enamel. Helv. odont. Acta8, 108–111 (1964).
Nancollas, G. H., Mohan, M. S.: The growth of hydroxyapatite crystals. Arch. oral Biol.15, 731–745 (1970).
Neuman, W. F., Neuman, M. W.: The chemical dynamics of bone mineral. Chicago: Chicago Univ. Press 1958.
Neuman, W. F., Neuman, M. W., Main, E. R., O'Leary, J., Smith, F. A.: The surface chemistry of bone. II. Fluoride deposition. J. biol. Chem.187, 655–661 (1950).
Neuman, W. F., Toribara, T. Y., Mulryan, B. J.: Synthetic hydroxyapatite crystals. I. Sodium and potassium fixation. Arch. Biochem.98, 384–390 (1962).
Posner, A. S.: Chapter 22, Phosphorus and its compounds, in Mineralized tissues, vol. II, Van Wazer, ed., p. 1442. New York: Interscience Publishing, Inc. 1961.
Posner, A. S., Perloff, A.: Apatites deficient in divalent cations. J. Res. Nat. Bureau Stds.58, 279–286 (1957).
Sarkar, B. C. R., Chauhan, U. P. S.: A new method for determining microquantities of calcium in biologic materials. Analyt. Biochem.20, 155–156 (1967).
Termine, J. D., Posner, A. S.: Calcium phosphate formationin vitro. I. Factors affecting initial phase separation. Arch. Biochem.140, 307–317 (1970).
Author information
Authors and Affiliations
Additional information
Support: Veterans Administration. P. H. S. Training Grant No. 5 T01 DE 00007-14.
Rights and permissions
About this article
Cite this article
Feagin, F.F., Gonzalez, M. & Jeansonne, B.G. Kinetic reactions of calcium, phosphate, and fluoride ions at the enamel surface-solution interface. Calc. Tis Res. 10, 113–127 (1972). https://doi.org/10.1007/BF02012541
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02012541