Abstract
Using intact and fractionated human polymorphonuclear leukocytes (PMNL), we provide evidence that the enantioselective leukotriene synthesis inhibitor (LSI) BAY X 1005, (R)-2-[4-(quinolin-2-yl-methoxy)phenyl]-2-cyclopentyl acetic acid binds specifically to a high-affinity binding site, which is most likely identical to FLAP.
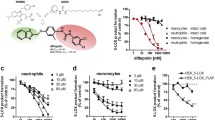
BAY X 1005 blocks the translocation of 5-lipoxygenase (5-LOX) in PMNL stimulated by the calcium ionophore A23187 or chemotactic stimuli such as PAF, C5a or fMLP as does MK-886. In contrast to the direct 5-LOX inhibitors (LOI) A-64077 and AA-861, the degree of leukotriene synthesis inhibition declines with increasing duration of A23187-induced leukocyte activation in the presence of BAY X 1005 and MK-886. Kinetic studies performed with BAY X 1005 showed that this effect was not accompanied by a significant translocation of 5-LOX from the cytosol to the microsomal fraction. Because FLAP has been implicated in the transfer of arachidonic acid to 5-LOX and A23187 is a potent activator of leukocyte phospholipase A2, we hypothesized that the observed loss of leukotriene synthesis inhibition may be due to competition of BAY X 1005 binding by endogenously released arachidonic acid. Accordingly, binding of BAY X 1005 to FLAP in intact and fractionated cells is dose-dependently inhibited by arachidonic acid and other unsaturated long-chain fatty acids, but not by saturated fatty acids. Therefore, we conclude that BAY X 1005 or MK-886 inhibit leukotriene biosynthesis by binding to FLAP, thereby preventing 5-LOX translocation and substrate transfer to the enzyme.
Similar content being viewed by others
References
J. A. Mancini, M. Abramovitz, M. E. Cox, E. Wong, S. Charleson, H. Perrier, Z. Wang, P. Prasit and P. J. Vickers,5-Lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett.318, 277–281 (1993).
A. Hatzelmann, R. Fruchtmann, K.-H. Mohrs, S. Raddatz and R. Müller-Peddinghaus,Mode of action of the new selective leukotriene synthesis inhibitor BAY X 1005, (R)-2-[4-(quinolin-2-yl-methoxy)phenyl]-2-cyclopentyl acetic acid, and structurally related compounds. Biochem. Pharmacol.45, 101–111 (1993).
R. Müller-Peddinghaus, C. Kohlsdorfer, P. Theisen-Popp, R. Fruchtmann, E. Perzborn, B. Beckermann, K. Bühner, H. J. Ahr and K.-H. Mohrs,BAY X 1005, a new inhibitor of leukotriene synthesis: In vivo inflammation pharmacology and pharmacokinetics. J. Pharmacol. Exp. Ther.267, 51–57 (1993).
S. Kargman, P. Prasit and J. EvansTranslocation of HL-60 cell 5-lipoxygenase. J. Biol. Chem.266, 23745–23752 (1991).
A. Wong, M. N. Cook, S. M. Hwang, H. M. Sarau, J. J. Foley and S. T. Crooke,Stimulation of leukotriene production and membrane translocation of 5-lipoxygenase by cross-linking of the IgE receptors in RBL-2H3 cells. Biochemistry31, 4046–4053 (1992).
A. Hatzelmann, R. Fruchtmann, K.-H. Mohrs, S. Raddatz, M. Matzke, U. Pleiss, J. Goosens, J. Keldenich and R. Müller-Peddinghaus,Mode of action of the leukotriene synthesis inhibitor BAY X 1005, and other structurally related quinoline derivatives—biological implications. In: Adv. Prostaglandins Leukotr. Research; Raven Press, New York (in press).
R. Fruchtmann, K.-H. Mohrs, A. Hatzelmann, S. Raddatz, B. Fugmann, B. Junge, H. Horstmann and R. Müller-Peddinghaus,In vitro pharmacology of BAY X 1005, a new inhibitor of leukotriene synthesis. Agents and Actions38, 188–195 (1993).
J. D. Winkler, C.-M. Sung, W. C. Hubbard and F. H. Chilton,Influence of arachidonic acid on indices of phospholipase A 2 activity in the human neutrophil. Biochem. J.291, 825–831 (1993).
S. Hammarström, M. Hamberg, B. Samuelsson, E. Duell, M. Stawiski and J. J. Voorhees,Increased concentration of non-esterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E, and prostaglandin F 2 alpha in epidermis of psoriasis. Proc. Natl. Acad. Sci. USA72, 5130–5134 (1975).
M. Abramovitz, E. Wong, M. E. Cox, C. D. Richardson and P. J. Vickers,5-Lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur. J. Biochem.215, 105–111 (1993).
M. Coffey, M. Peters-Golden, J. C. Fantone III and P. H. S. Sporn,Membrane association of active 5-lipoxygenase in resting cells—Evidence for novel regulation of the enzyme in the rat alveolar macrophage. J. Biol. Chem.267, 570–576 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kast, R., Fruchtmann, R., Kupferschmidt, R. et al. Role of 5-lipoxygenase-activating protein in the regulation of 5-lipoxygenase activity in human neutrophils. Agents and Actions 41 (Suppl 2), C166–C168 (1994). https://doi.org/10.1007/BF01987624
Issue Date:
DOI: https://doi.org/10.1007/BF01987624