Abstract
Germination of chlamydospores ofFusarium oxysporum f. sp.pisi race 1 in the rhizosphere of pea seedlings and red clover seedlings grown in natural soil heavily infested with the pathogen, was highest in percentage along the actively growing parts of the roots. At these sites, exudation of ninhydrinpositive substances and reducing sugars was most intense with seedlings grown in vitro.
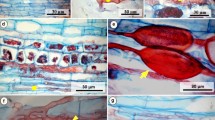
No significant difference in the percentage of germinating chlamydospores ofFusarium oxysporum f. sp.pisi race 1 were observed in the rhizosphere soil and on the root surface of homologous parts of roots of seedlings and mature plants of a susceptible ‘Rondo’ and a resistant ‘Rovar’ pea cultivar grown in natural soil heavily infested with the pathogen. Differences in the growth of mycelium of the pathogen on the root surface, or in the attachment of the mycelium to the root surface of both cultivars were not observed. Epidermis and cortex cells of roots of both cultivars reacted on penetration by the pathogen by producing a cellulose thickening of the cell wall, which later became infiltrated with a ligning-like material. A selective effect on the activities of the pathogen in the rhizosphere, on the root surface and in the epidermis and cortex in relation to resistance thus could not be demonstrated. Formation of new chlamydospores from germ tubes of germinating chlamydospores was frequently observed in the rhizosphere of the susceptible and resistant pea cultivar and in the rhizosphere of red clover seedlings.
Similar content being viewed by others
References
Buxton, E. W., 1957a. Some effects of pea root exudates on physiologic races of Fusarium oxysporum Fr. f. pisi (Linf.) Snyder & Hansen, Trans. Br. mycol. Soc. 40: 145–154.
Buxton, E. W., 1957b. Differential rhizosphere effects on three pea cultivars on physiologic races of Fusarium oxysporum f. pisi. Trans. Br. mycol. Soc. 40: 305–316.
Buxton, E. W., 1960. Effects of pea root exudates on the antagonism of some rhizosphere microorganisms towards Fusarium oxysporum f. pisi. J. gen. Microbiol. 22: 678–689.
Flentje, N. T., 1947. A study of pre-emergence rotting in wrinkled seeded peas. M. Sc. thesis, Univ. of Adelaide, South Australia.
Flentje, N. T., 1959. The physiology of penetration and infection. pp. 76–87. In: C. S. Holten et al. (Editors): Plant pathology: Problems and progress 1908–1958. University of Wisconsin Press, Madison.
Flentje, N. T. & Saksena, H. K., 1964. Pre-emergence rotting of peas in South Australia. III. Hostpathogen interaction. Aust. J. biol. Sci. 17: 665–675.
Fuchs, A., Rohringer, R. & Samborski, D. J., 1967. Metabolism of aromatic compounds in healthy and rust-infected primary leaves of wheat. II. Studies with L-phenyl alanine-U-14C, L-tyrosine-U-14C, and ferulati-U-14C. Can. J. Bot. 45: 2137–2153.
Fuchs, A. & Vries, F. W. de, 1969. Metabolism of radioactively labeled quinic acid and shikimic acid in healthy and Fusarium-infected tomato plants. Neth. J. Pl. Path. 75: 186–192.
griffiths, D. A. & Lim, W. E., 1964. Mechanical resistance in root hairs to penetration by species of vascular wilt fungi. Mycopath. Mycol. appl. 24: 103–112.
Husain, S. S. & McKeen, W. E., 1963. Interactions between strawberry roots and Rhizoctonia fragariae. Phytopathology 53: 541–545.
Hywegen, T., 1963. Lignification, a possible mechanism of active resistance against pathogens. Neth. J. Pl. Path. 69: 314–317.
Johansen, D. A., 1940. Plant microtechnique. McGraw-Hill, New York, 523 pp.
Kommedahl, T., 1966. Relation of exudates of pea roots to germination of spores in races of Fusarium oxysporum f. pisi. Phytophathology 56: 721–722.
Partridge, S. M., 1946. Application of the paper partition chromatogram to the qualitative analysis of reducing sugars. Nature, Lond. 158: 270–271.
Porter, C. A., Margolis, D. & Sharp, P., 1957. Quantitative determinations of amino acids by paper chromatography. Contr. Boyce Thompson Inst. Pl. Res. 18: 465–476.
Rajagopalan, K. & Bhuvaneswari, K., 1964. Effect of germination of seeds and host exudations during germination on foot-rot disease of rice. Phytopath. Z. 50: 221–226.
Rohringer, R. & Samborski, D. J., 1967. Aromatic compounds in the host-parasite interaction. A. Rev. Phytopath. 5: 77–86.
Rovira, A. D., 1959. Root excretions in relation to the rhizosphere effect. IV. Influence of plant species age of plant, light, temperature, and calcium nutrition on exudation, Pl. Soil. 11: 53–64.
Schippers, B., 1967. De kieming van chlamydosporen van Fusarium oxysporum f. pisi ras 1 in de rhizosfeer van een resistente en een vatbare erwtenvariëteit. Neth. J. Pl. Path. 73: 68. (Abstr.).
Schroeder, W. T. & Walker, J. C., 1942. Influence of controlled environment and nutrition on the resistance of garden pea to Fusarium wilt. J. agric. Res. 65: 221–248.
Schroth, M. N. & Cook, R. J., 1964. Seed exudation and its influence on pre-emergence damping-off of bean. Phytopathology 54: 670–673.
Schroth, M. N. & Hendrix, F. F. Jr., 1962. Influence of non-susceptible plants on the survival of Fusarium solani f. phaseoli in soil. Phytopathology 52: 906–909.
Schroth, M. N. & Hildebrand, D. C., 1964. Influence of plant exudates on root-infecting fungi. A. Rev. Phytopath. 2: 101–132.
Schroth, M. N. & Snyder, W. C., 1961. Effect of host exudates on chlamydospore germination of the bean root rot fungus, Fusarium solani f. phaseoli. Phytopathology 51: 389–393.
Schroth, M. N., Weinhold, A. R. & Hayman, D. S. 1966. The effect of temperature on quantitative differences in exudates from germinating seeds of bean, pea, and cotton. Can. J. Bot. 44: 1429–1432.
Stoughton, R. H., 1929. Thionin and orange-G for the differential staining of bacteria and fungi in plant tissues. Ann. appl. Biol. 17: 162–165.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schippers, B., Voetberg, J.S. Germination of chlamydospores of Fusarium oxysporum f. sp. pisi race 1 in the rhizosphere, and penetration of the pathogen into roots of a susceptible and a resistant pea cultivar. Netherlands Journal of Plant Pathology 75, 241–258 (1969). https://doi.org/10.1007/BF01981515
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01981515