Abstract
We have used the carrageenan-induced pouch-granuloma in rats to investigate how changes in low-molecular-mass iron chelate levels in the exudate, induced by iron loading (iron-dextran) or chelation (desferrioxamine) influence cellular and systemic inflammatory parameters.
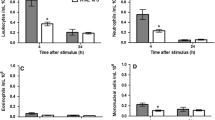
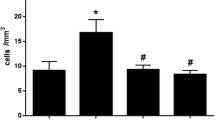
In the iron-treated group we observed a rapid decrease in the number of leukocytes and exudate volume; there was also an increase in ferritin iron and low-molecular-mass iron chelates, and on the eighth day a systemic response. In the desferrioxamine-treated group we detected a decrease in low-molecular-mass iron chelates, ferritin iron, and an increase in the number of leukocytes. We describe the protective effects of desferrioxamine against the deleterious effects of ferrous iron and relate this to its chelating and scavenging activity.
The results suggest that the levels of low-molecular-mass iron chelates modulate the inflammatory response, possibly through their contribution to the oxygen free radical generation, which is responsible for the cell membrane damage and subsequently its death. The modulatory action of iron-dextran and desferrioxamine support our hypothesis.
Similar content being viewed by others
References
P. Puig-Parellada and J. M. Planas,Synovial fluid degradation induced by free radicals. In vitro action of several free radical scavengers and anti-inflammatory drugs. Biochem. Pharmacol.27, 535–537 (1978).
F. A. Jr. Kuehl, J. L. Humes, M. L. Torchiana, E. A. Ham and R. W. Egan,Oxygen-centered radicals in inflammatory processes. Adv. Inflamm. Res.1, 419–430 (1979).
J. M. C. Gutteridge, D. A. Rowley and B. Halliwell,Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of “free” iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem. J.199, 263–265 (1981).
P. Biemond, H. G. van Eyk, A. J. G. Swaak and J. F. Koster,Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J. Clin. Invest.73, 1576–1579 (1984).
B. Halliwell, J. M. C. Gutteridge and D. Blake,Metal ions and oxygen radical reactions in human inflammatory joint disease. Phil. Trans. R. Soc. Lond. B311, 659–671 (1985).
J. M. C. Gutteridge,Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett.201, 291–295 (1986).
D. R. Blake, N. D. Hall, P. A. Bacon, P. A. Dieppe, B. Halliwell and J. M. C. Gutteridge,The importance of iron in rheumatoid diseases.Lancet ii, 1142–1144 (1981).
M. C. Linder and H. N. Munro,Assay of tissue ferritin. Anal. Biochem.48, 266–278 (1972).
T. L. Dormandy,Caeruloplasmin: acute-phase antioxidant.In Trace elements on the pathogenesis and treatment of inflammation. (Ed. K. D. Rainsford, K. Brune and M. W. Whitehouse) pp. 185–197, Birkhäuser Verlag, Basel 1981.
S. Osaki, D. A. Johnson and E. Frieden,The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J. Biol. Chem.241, 2746–2751 (1966).
I. M. Goldstein, H. B. Kaplan, H. S. Edelson and G. Weisseman,Ceruloplasmin-A scavenger of superoxide anion radicals. J. Biol. Chem.254, 4040–4045 (1979).
M. Fukuhara and S. Tsurufuji,The effect of locally injected anti-inflammatory drugs on the carrageenin granuloma in rats. Biochem. Pharmacol.18, 475–484 (1969).
J. W. Drysdale and W. N. M. Ramsay,The separation of ferritin and haemosiderin for studies in the metabolism of iron. Biochem. J.95, 282–288 (1965).
F. W. Jr. Sunderman and S. Nomoto,Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin. Chem.16, 903–910 (1970).
J. Ball, J. A. Chapman and K. D. Muirden,The uptake of iron in rabbit synovial tissue following intra-articular injection of iron dextran. A light and electron microscope study. J. Cell Biol.22, 351–364 (1964).
B. S. van Asbeck, J. J. M. Marx, A. Struyvenberg, J. H. van Kats and J. Verhoef,Effect of iron (III) in the presence of various ligands on the phagocytic and metabolic activity of human polymorphonuclear leukocytes. J. Immunol.132, 851–856 (1984).
A. G. Mowat, T. F. Disney and J. H. Vaughan,Effect of iron dextran, gold thiosulphate and hydrocortisone acetate on experimental synovitis in the guinea-pig. Ann. Rheum. Dis.30, 187–193 (1971).
P. G. Winyard, D. R. Blake, S. Chirico, J. M. C. Gutteridge and J. Lunec,Mechanism of exacerbation of rheumatoid synovitis by total-dose iron-dextran infusion: in-vivo demonstration of iron-promoted oxidant-stress. Lancet 69–72 (1987).
V. M. Samokyszyn, D. M. Miller, D. W. Reif and S. D. Aust,Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J. Biol. Chem.264, 21–26 (1989).
I. M. Hoepelman, E. Y. Jaarsma, J. Verhoef and J. J. M. Marx,Effect of iron on polymorphonuclear granulocyte phagocytic capacity: role of oxidation state and effect of ascorbic acid. Br. J. Haematol.70, 495–500 (1988).
G. Curzon and S. O'Reilly,A coupled iron-caeruloplasmin oxidation system. Biochem. Biophys. Res. Com.2, 284–286 (1960).
E. Graf, J. R. Mahoney, R. G. Bryant and J. W. Eaton,Iron catalyzed hydroxyl radical formation. J. Biol. Chem.259, 3620–3624 (1984).
J. Sinaceur, C. Ribière, J. Nordmann and R. Nordmann,Desferrioxamine: a scavenger of superoxide radicals? Biochem. Pharmacol.33, 1693–1694 (1984).
D. R. Blake, N. D. Hall, P. A. Bacon, P. A. Dieppe, B. Halliwell and J. M. C. Gutteridge,Effect of a specific iron chelating agent on animal models of inflammation. Ann. Rheum. Dis.42, 89–93 (1983).
F. J. Andrews, C. J. Morris, G. Kondratowicz and D. R. Blake,Effect of iron chelation on inflammatory joint disease. Ann. Rheum. Dis.46, 327–333 (1987).
S. Yoshino, D. R. Blake and P. A. Bacon,The effect of desferrioxamine on antigen-induced inflammation in the rat air pouch. J. Pharm. Pharmacol.36, 543–545 (1984).
M. T. Mitjavila, M. T. Carbonell, M. P. Sáiz, J. Muntané, J. Sánchez and P. Puig-Parellada,Role of iron in carrageenan-induced granuloma: Action of desferrioxamine and indometacin. Pharmacol.40, 236–240 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muntane, J., Fritsch, P., Carbonell, T. et al. Modulation of exudate inflammation parameters in rat carrageenan-induced granuloma by modification of exudate iron levels. Agents and Actions 32, 167–172 (1991). https://doi.org/10.1007/BF01980869
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01980869