Abstract
Particle counts were performed on 1,000 intravenous administration sets from ten suppliers on the Swiss market using a HIAC/Royco electronic counter. The following main conclusions may be drawn from the results of this study:
-
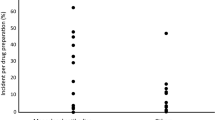
the small particles were the most numerous, regardless of the type of set;
-
the differences in the counts obtained for the different suppliers' sets tended to level off for particles larger than 10μm;
-
the drip chamber and latex connector may be two important sources of particles;
-
the particulate contamination from the sets is relatively low compared with the amount of particles contained in the parenteral solutions.
Similar content being viewed by others
References
Akers MJ. Parenteral quality control. New York: Marcel Dekker, 1985:143–97.
De Luca PP, Knapp JZ. Particulate matter. In: Avis KE, Lachman L, Lieberman HA, eds. Pharmaceutical dosage forms. Parenteral medication. New York: Marcel Dekker, 1984:217–307.
Anonymous. I.V. filters. Med Lett Drug Ther 1975;17:35–6.
Schubert GE, Reifferscheid P, Flach A. Mikroembolien von Fremdmaterial nach Angiographie und intravenösen Infusionen. Dtsch Med Wochenschr 1972;97:1745–8.
Dimmick JE, Bove KE, Mc Adams J, Benzing G. Fiber embolization: a hazard of cardiac surgery and catheterization. N Engl J Med 1975;292:685–7.
Klaus E. Materielle Verunreinigung in Infusionslösungen. Klin Anästh Intensivther 1977;14:54–69.
Ahnefeld FW, Klaus E. Quantitative Analyse über den Partikelgehalt von Infusionslösungen,-zubehör und Medikamenten. Anaesthesist 1977;26:476–84.
Gesler RM, Garvin PJ, Klamer B, et al. The biological effects of polystyrene latex particles administered intravenously to rats. Bull Parenter Drug Assoc 1973;27:101–13.
Schröder HG, Simmons GH, De Luca PP. Distribution of radiolabeled subvisible microspheres after intravenous administration to Beagle dogs. J Pharm Sci 1978;67:504–7.
Kanke M, Simmons GH, Weiss DL, Bivins BA, De Luca PP. Clearance of141Ce-labeled microspheres from blood and distribution in specific organs following intravenous and intraarterial administration in Beagle dog. J Pharm Sci 1980;69:755–62.
Ryan PB, Rapp RP, De Luca PP, Griffen WO, Clark JD, Cloys D. In-line final filtration. A method of minimizing contamination in intravenous therapy. Bull Parenter Drug Assoc 1973;27:1–14.
Bivins BA, Rapp RP, De Luca PP, Mc Kean H, Griffen WO. Final in-line fitraton: a mean of decreasing the incidence of infusion phlebitis. Surgery 1979;85:388–94.
Anonymous. Pharmacopoea Helvetica VII. Parenteralia. Bern: 1987.
Anonymous. Pharmacopée Européenne. Parenteralia. Ste-Ruffine: Maisonneuve SA, 1977:414.
Anonymous. United States Pharmacopeia XXI. Rockville: United States Pharmacopeial Convention, 1985.
Ho NFH, Church RL, Lee H. Particulate matter in parenteral solutions. II. Drug Intell 1967;1:356–61.
Williams A, Barnett MI. Particulate matter in intravenous fluids, administration sets and cannulae. Pharm J 1973;211:190–1.
Olson OT. Particulate generation from equipment used for the administration of infusion fluids. Acta Pharm Suec 1980;17:209–14.
Harrison MJ, Healy TEJ. Intravenous administration sets: the effect of flushing and filtration on particulate contamination. Br J Anaesth 1974;46:59–65.
Pavanetto F, Conti B, Modena T, Genta I, Ponci R. Particulate contamination in parenteral type medical device. Int J Pharm 1988;48:255–265.
Lantz RJ, Shami EG, Lachmann L. Application of the HIAC particle counter to monitoring particulate matter in parenteral solutions I. Bull Parenter Drug Assoc 1976;30:234–46.
Schröder HG, De Luca PP. Theoretical aspects of particulate matter monitoring by microscopic and instrumental methods. J Parenter Drug Assoc 1980;34:183–9.
Winer DJ. Statistical principles in experimental dessign. New York: McGraw Hill, 1971.
Bolton S. Pharmaceutical statistics. New York: Marcel Dekker, 1984.
Anonymous. Deutsches Institut für Normung (DIN). 58 362/1. Berlin: Beuth Verlag, 1988.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Di Paolo, E.R., Hirschi, B. & Pannatier, A. Quantitative determination of particulate contamination in intravenous administration sets. Pharmaceutisch Weekblad Scientific Edition 12, 190–195 (1990). https://doi.org/10.1007/BF01980045
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01980045