Abstract
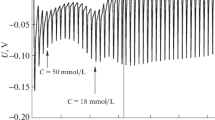
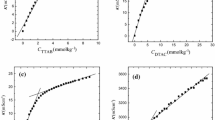
Densities, heat capacities, enthalpies of dilution at 298 K and osmotic coefficients at 310 K of octyltrimethylammonium chloride were measured as functions of concentration. From the experimental data, the partial molar volumes, heat capacities, relative enthalpies, nonideal free energies and entropies at 298 K were derived as functions of concentration. A comparison between the above data and those of dodecyltrimethylammonium chloride reported in the literature shows that the increase of the alkyl chain length shifts the apparent molar volumevs. concentration curves towards greater values and the heat capacity, relative enthalpy and free energyvs. concentration curves towards smaller values. By assuming the pseudo-phase transition model the properties of micellization (ΔYm) were graphically evaluated. TheΔYm values of OTAC compared with those of DTAC are consistent with the increase of the hydrophobicity by increasing the alkyl chain length.
Zusammenfassung
Für Oktyltrimethylammoniumchlorid wurden Dichte, Wärmekapazität, Lösungsenthalpie bei 298 K und osmotische Koeffizienten bei 310 K als Funktion der Konzentration gemessen. Anhand der experimentellen Daten worden die partiellen Molvolumen, Wärmekapazitäten, relativen Enthalpien, die nichtidealen freien Energien und Entropien bei 298 K als Funktionen der Konzentration erhalten. Ein Vergleich obiger Daten mit den in der Literatur beschriebenen Daten für Dodecyltrimethylammoniumchlorid zeigt, daß längere Alkylketten die Kurve (scheinbares Molvolumen/Konzentration) zu größeren Werten hin verschiebt, die entsprechenden Kurven von Wärmekapazität, relativer Enthalpie und freien Energien hin zu kleineren Werten. Unter Annahme des Pseudeophasenübergangsmodelles konnten das Mizellisationsvermögen (ΔYm)graphisch ermittelt werden. DieΔYm-Werte für OTAC entsprechen — verglichen mit denen von DTAC — dem Anstieg der Hydrophobizität bei anwachsender Alkylkettenlänge.
Similar content being viewed by others
References
R. De Lisi, S. Milioto and R. Triolo, J. Solution Chem., 17 (1988) 673.
R. De Lisi, E. Fisicaro and S. Milioto, J. Solution Chem., 17 (1988) 1015.
S. Causi, R. De Lisi and S. Milioto, J. Solution Chem., 20 (1991) 1031.
S. Milioto, S. Causi and R. De Lisi, J. Solution Chem. (in press).
R. De Lisi and S. Milioto, J. Solution Chem., 17 (1988) 245.
R. De Lisi, S. Milioto, A. Castagnolo and A. Inglese, J. Solution Chem., 19 (1990) 767.
R. De Lisi, S. Milioto, A. Castagnolo and A. Inglese, J. Phys. Chem., 95 (1991) 3322.
R. De Lisi, S. Milioto and R. E. Verrall, J. Solution Chem., 19 (1990) 639.
G. S. Kell, J. Chem. Eng. Data, 12 (1967) 66.
J. E. Garrod and T. M. Herrington, J. Phys. Chem., 74 (1970) 363.
M. F. Stimson, Am. J. Phys., 23 (1955) 614.
R. De Lisi, E. Fisicaro, S. Milioto, E. Pelizzetti and P. Savarino, J. Solution Chem., 19 (1990) 247.
R. De Lisi, C. Ostiguy, G. Perron and J. E. Desnoyers, J. Colloid Interface Sci., 71 (1979) 147.
C. Jolicoeur and G. Lacroix, Can. J. Chem., 54 (1976) 624.
J. E. Desnoyers, R. De Lisi, C. Ostiguy and G. Perron, Solution Chemistry of Surfactants, Vol. 1, K. L. Mittal Ed., Plenum, N.Y. 1979.
Author information
Authors and Affiliations
Additional information
The authors are grateful to the National Research Council of Italy (CNR, Progetto Finalizzato Chimica Fine II) and to the Ministry of University and of Scientific and Technological Research (MURST) for financial support.
Rights and permissions
About this article
Cite this article
Milioto, S., Causi, S., Crisantino, R. et al. Thermodynamic studies of octyltrimethylammonium chloride in water. Journal of Thermal Analysis 38, 2693–2705 (1992). https://doi.org/10.1007/BF01979745
Issue Date:
DOI: https://doi.org/10.1007/BF01979745