Abstract
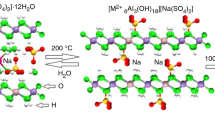
The rate of removal and uptake of guests into layered and porous materials is important in many areas of materials chemistry. Here we report on the use of atmospheric thermogravimetry linked to a mass spectrometer (TG-MS) to investigate the thermal characteristics of three different solids. We show that (i) the desorption of cyclohexylamine from the surface of a pillared acid activated smectite clay occurs in two stages, indicating two possible acidic binding sites, (ii) TG-MS is an extremely sensitive technique for probing progressive anion exchange of lithium aluminium layered double hydroxides, (iii) on heating, the perhydrate 4Na2SO4·NH4Cl·2H2O2 releases H2O2 intact.
Similar content being viewed by others
References
D. Dollimore, G. A. Gamlen and T. J. Taylor, Thermochim. Acta, 75 (1984) 59.
J. P. Redfern, Polym. Int. 26 (1991) 51; J. Appl. Polym. Sci.: Appl. Polym. Symp., 55 (1994) 65.
T. J. Pinnavaia, Science, 220 (1983) 365.
F. Fueras, Catal. Rev., Sci. Eng., 30 (1988) 457.
J. Bovey, E. Fowles, W. Jones, R. Mokaya and M. E. Davies, GB Patent No. PCT/GB94/02480 (1995).
M. Frenkal, Clays Clay Minerals, 22 (1975) 435.
C. Breen, Clay Minerals, 26 (1991) 473.
C. J. Serna, J. L. Rendon and J. E. Iglesias, Clays Clay Mineral., 30 (1982) 180.
F. Cavani, F. Trifir and A. Vaccari, Catal. Today, 11 (1991) 173.
I. C. Chisem and W. Jones, J. Mater. Chem., 4 (1994) 1737.
A. K. Galwey and W. J. Hood, J. Phys. Chem., 83 (1979) 1810; J. Chem. Soc. Faraday, 72 (1982) 2815.
J. M. Adams, V. Ramdas, G. G. T. Guarini and C. J. Adams, J. Chem. Soc. Dalton Trans., (1980) 269.
J. M. Adams, R. G. Pritchard and J. M. Thomas, J. Chem. Soc. Chem. Comm., (1978) 288.
Blendax-Werke R Schneider GmbH & Co., Euro. Pat. Appl. EP257 399 (1988).
S. D. Cosgrove and W. Jones, unpublished.
S. D. Cosgrove and W. Jones, J. Chem. Soc. Chem. Comm., 19 (1994) 2255.
J. Bovey and W. Jones, J. Mater. Chem., 5 (1995) 2027.
J. A. Ballantine, P. Graham, I. Patel, J. H. Purnell, K. J. Williams and J. M. Thomas, Proc. Int. Clay Conf. Denver, (1985) 311.
R. S. Weber, P. Gallezot, F. Lefebvre and S. L. Suib, Microporous Mater., 1 (1993) 223.
Author information
Authors and Affiliations
Additional information
We are very grateful to Miss J. Bovey for supplying us with the acid activated clay and for very helpful comments. We would like to thank Rheometric Scientific Ltd. and Leda Mass Ltd. for their advice and co-operation in designing and fitting the transfer line. Financial assistance from the EPSRC through a Quota award to ICC and a CASE award with Solvay Interox Ltd. to SDC is also appreciated.
Rights and permissions
About this article
Cite this article
Chisem, I.C., Cosgrove, S.D. & Jones, W. Studies of layered and channel complexes using combined thermogravimetry and mass spectrometry. Journal of Thermal Analysis 50, 757–766 (1997). https://doi.org/10.1007/BF01979205
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01979205