Abstract
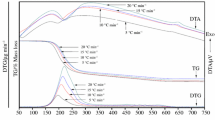
The thermal decomposition of [Co(NH3)6]2(C2O4)3·4H2O was studied under isothermal conditions in flowing air and argon. Dissociation of the above complex occurs in three stages. The kinetics of the particular stages thermal decomposition have been evaluated. The RN and/or AM models were selected as those best fitting the experimental TG curves. The activation energies,E, and lnA were calculated with a conventional procedure and by a new method suggested by Kogaet al. [10, 11]. Comparison of the results have showed that the Arrhenius parameters values estimated by the use of both methods are very close. The calculated activation energies were in air: 96 kJ mol−1 (R1.575, stage I); 101 kJ mol−1 (Ain1.725 stage II); 185 kJ mol−1 (A 2.9, stage III) and in argon: 66 kJ mol−1 (A 1.25, stage I); 87 kJ mol−1 (A 1.825, stage II); 133 kJ mol−1 (A 2.525, stage III).
Similar content being viewed by others
References
W. W. Wendlandt, Thermal Analysis, Wiley, New York, 3rd edn., 1986, Chapt. 2, 5, 8.
E. L. Charsley and S. B. Warrington (Eds), Thermal Analysis — Techniques and Applications, Royal Society of Chemistry, Cambridge 1992, p. 1–156.
F. Paulik, Special Trends in Thermal Analysis, Wiley, New York 1995.
W. E. Brown, D. Dollimore and A. K. Galwey, in C. H. Bamford and C. F. H. Tipper (Eds), Comprehensive Chemical Kinetics, Vol. 22, Elsevier, Amsterdam 1980.
M. Reading, Thermochim. Acta, 135 (1988) 37.
J. Rouquerol, Thermochim. Acta, 144 (1989) 209.
J. H. Sharp, G. W. Brindley and B. N. N. Achar, J. Amer. Ceram. Soc., 49 (1966) 379.
C. Różycki and M. Maciejewski, Thermochim. Acta, 96 (1985) 91.
N. J. Carr and A. K. Galwey, Thermochim. Acta, 79 (1984) 323.
N. Koga, J. Šesták and J. Malek, Thermochim. Acta, 188 (1991) 333.
N. Koga and H. Tanaka, J. Thermal Anal., 41 (1994) 455.
R. Ozao and M. Ochiai, J. Thermal Anal., 40 (1993) 1331.
E. Ingier-Stocka, in preparation.
E. Ingier-Stocka, J. Thermal Anal., 40 (1993) 1357.
Inorg. Synt., 2 (1946) 220.
E. Ingier-Stocka, Thermochim. Acta, 170 (1990) 107.
M. Maciejewski and A. Reller, Thermochim. Acta, 110 (1987) 145.
H. Tanaka, Thermochim. Acta, 48 (1981) 137.
A. K. Galwey, S. G. McKee and T. R. B. Mitchell, Reactivity of Solids, 6 (1988) 173.
A. K. Galwey, Thermochim. Acta, 96 (1985) 259.
A. Coetzee, M. E. Brown, D. J. Eve and C. A. Strydom, J. Thermal Anal, 41 (1994) 357.
A. Venkataraman, N. V. Sastry and A. Ray, J. Phys. Chem. Solids, 53 (1992) 681.
M. E. Brown, D. Dollimore and A. K. Galwey, Thermochim. Acta, 21 (1977) 103.
D. Dollimore, J. Dollimore and J. Little, J. Chem. Soc. A (1969) 2946.
A. Alloun and C. G. R. Nair, Thermochim. Acta, 92 (1985) 767.
B. Topley and M. L. Smith, J. Chem. Soc., (1935) 321.
J. Błażejowski, Thermochim. Acta, 76 (1984) 359.
H. M. Fogel and J. E. House, Jr, J. Thermal Anal., 34 (1988) 231.
C. G. R. Nair and S. Mathew, Thermochim. Acta, 150 (1989) 63.
A. Taskinen, P. Taskinen and M. H. Tikkanen, Reactivity of Solids, Proc. 8th Int. Symp. Plenum, New York 1977, p. 617.
D. Dollimore and T. A. Evans, Thermochim. Acta, 178 (1991) 263.
H. Jost, J. Jedamzik, M. Rossberg and Ch. Staedler, Z. Phys. Chemie, Leipzig, 266 (1985) 311.
S. A. Jones, J. Pearce and J. Fenerty, Proc. 9th ICTA Congress 1988 (Abstr.) p. 176.
D. Dollimore, T. A. Evans and Y. F. Lee, Thermochim. Acta, 194 (1992) 215.
P. E. Yankwich and P. D. Zavitsanos, Pure Appl. Chem., 8 (1964) 287.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ingier-Stocka, E. Kinetics of the thermal decomposition of [Co(NH3)6]2(C2O4)3·4H2O. Journal of Thermal Analysis 50, 603–616 (1997). https://doi.org/10.1007/BF01979032
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01979032