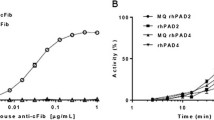
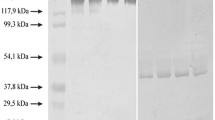
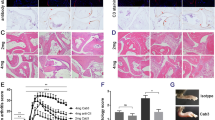
Abstract
The function of α2M (α2-macroglobulin) as a proteinase inhibitor in synovial fluid of rheumatoid arthritis was investigated. Low esterase activity was found in the 19S sieve fraction of Sephadex G-200 gel filtered synovial fluid samples, comparable with that obtained with normal human plasma. The molar binding ratio of α2M isolated from the synovial fluid and trypsin was estimated according to earlier established methods. The formation of an equimolar complex indicated that the synovial α2M was functionally intact.
Esterase activities were measured on αN-tosyl-l-arginine [3H]methyl ester. Synovial fluid samples were all found to contain proteinase enzyme which did not bind to the synovial α2M nor to the added functionally intact, immunologically pure α2M prepared from normal human plasma.
Albumin, α1-acid glycoprotein, α2HS-glycoprotein, α2M and kininogen in synovial fluids were determined by single radial immunodiffusion. Only the α2M contents were clearly lower than in normal human serum per ml.
The higher than 1 ratio between the immunoreactive kininogen determined with monospecific anti-human kininogen serum and the pharmacologically active kininogen indicated that kinin release had occurred possibly in the synovial membrane. Proteinase enzymes present in the synovial fluids and unbound to α2M caused further depletion of the active kinin segment in synovial kininogen. A model of the regulation by α2M of the synovial kininogen-kinin system is given.
Similar content being viewed by others
References
A.J. Barrett andP.M. Starkey, The Interaction of α2-Macroglobulin with Proteinases. Characteristics and specificity of the Reaction, and a Hypothesis Concerning its Molecular Mechanism, Biochem. J.133, 709–724 (1973).
C.-B. Laurell andJ.-O. Jeppsson,Protease Inhibitors in Plasma, in:The Plasma Proteins, Vol. I (Ed. F.W. Putnam; Academic Press, New York, San Francisco, London, 1975), pp. 229–264.
U. Hamberg, P. Stelwagen andH.-S. Ervast, Human α-Macroglobulin and Trypsin and Binding, Purification Methods, Trypsin and Plasmin Complex Formation, Eur. J. Biochem.40, 439–451 (1973).
G. Shtacher, R. Maayan andG. Feinstein,Proteinase Inhibitors in Human Synovial Fluid, Biochim. Biophys. Acta303, 138–147 (1973).
G. Feinstein, G. Shtacher andR. Maayan,Protease Inhibitors in Human Synovial Fluids of Patients with Joint Diseases, in:Proteinase Inhibitors.Proceedings of the 2nd International Research Conference (Eds. H. Fritz, H. Tschesche, L.J. Geene and E. Truscheit; Springer-Verlag, Berlin, Heidelberg, New York, 1974), pp. 109–110.
U. Hamberg, Regulation of Kininogenase Action by Human α2-Macroglobulin, abstract in:IVth International Congress on Thrombosis and Haemostasis, p. 126 (1973).
M.E.J. Billingham andA.H. Gordon,The Role of the Acute Phase Reaction in Inflammation, Agents and Actions6, 195–199 (1976).
J.N. Sharma, I.J. Zeitlin, P.M. Brooks andW.C. Dick,A Novel Relationship between Plasma Kininogen and Rheumatoid Disease, Agents and Actions6, 148–153 (1976).
U. Hamberg andTh. Tallberg,Preparation of Monospecific Antiserum to Human LMW Kinonogen using Immunoadsorbents, J. Immunol. Methods2, 17–24 (1972).
U. Hamberg, P. Elg, E. Nissinen andP. Stelwagen,Purification and Heterogeneity of Human Kininogen. Use of DEAE-chromatography, Molecular Sieving and Antibody Specific Immunosorbents, Int. J. Peptide Protein Res.7, 261–280 (1975).
V.H. Beaven, J.V. Pierce andJ.J. Pisano,A Sensitive Isotopic Procedure for the Assay of Esterase Activity: Measurement of Human Urinary Kallikrein, Clin. Chim. Acta32, 67–73 (1971).
B.F. Erlanger, N. Kokowsky andW. Cohen,The Preparation and Properties of Two New Chromogenic Substrates of Trypsin, Arch. Biochem. Biophys.95, 271–278 (1961).
C. Kutzbach andG. Schmidt-Kastner,Properties of Highly Purified Hog Pancreatic Kallikrein, in:Kininogenases. Kallikrein. 1st Symposium on Physiological Properties and Pharmacological Rational (Eds. G.L. Haberland and J.W. Rohen; F.K. Schattauer Verlag, Stuttgart, New York, 1973), pp. 23–35.
G. Mancini, A.O. Carbonara andJ.F. Heremans,Immunochemical Quantitation of Antigens by Single Radial Immunodiffusion, Immunochem.2, 235–254 (1965).
E.A. Kabat,Precipitin Reaction, in:Kabat and Mayer's Experimental Immunochemistry (C.C. Thomas, Springfield, 1961), pp. 69–70.
Ö. Ouchterlony,Antigen-Antibody Reactions in Gels, Arkiv. för Kemi, Mineralogi och Geologi, Bd.26B, 1–9 (1948).
O.H. Lowry, N.J. Roseborough, A.L. Farr andR.J. Randall,Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem.193, 265–275 (1951).
H.E. Schultze andJ.F. Heremans,Molecular Biology of Human Proteins with Special Reference to Plasma Proteins, vol. 1 (Elsevier Publishing Company, Amsterdam, London, New York, 1966).
U. Hamberg,Kininogen in Human Plasma after Fibrinolytic Activation, Scand. J. Clin. Lab. Invest.24, Suppl. 107, 37–47 (1969).
U. Hamberg, P. Elg andP. Stelwagen,Tryptic and Plasmic Peptide Fragments Increasing the Effect of Bradykinin on Isolated Smooth Muscle, Scand. J. Clin. Lab. Invest.24, Suppl. 107, 21–35 (1969).
U. Ragnarsson, S.M. Karlsson andU. Hamberg,Synthesis of Peptides by Fragment Condensation on a Solid Support. 1. Application in Preparation of Bradykinin, Int. J. Peptide Protein Res.7, 307–312 (1975).
R.A. Jessar,The Study of Synovial Fluid, in:Arthritis and Allied Conditions (Eds. Hollander and McCarty, Jr; Lea and Febiger Philadelphia, 1972).
F.W. Putnam, in:The Plasma Proteins, vol. I. (Academic Press, New York, San Francisco, London, 1975), pp. 26–27, 60.
E. Vahtera andU. Hamberg, Absence of Binding of Pancreatic and Urinary Kallikreins to α2-Macroglobulin, Biochem. J.157, 521–524 (1976).
Z. Werb, M.C. Burleigh, A.J. Barrett andP.M. Starkey, The Interaction of α2-Macroglobulin with Proteinases. Binding and Inhibition of Mammalian Collagenases and Other Metal Proteinases, Biochem. J.139, 359–368 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamberg, U., Vahtera, E. & Moilanen, L. Functionally active α2-macroglobulin and kinin release in synovial fluids of rheumatoid arthritis. Agents and Actions 8, 50–56 (1978). https://doi.org/10.1007/BF01972402
Issue Date:
DOI: https://doi.org/10.1007/BF01972402