Abstract
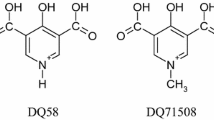
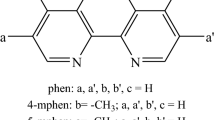
The metal complexing properties of two antihypertensive drugs, hydralazine (1-hydrazinophthalazine) and prizidilol (a hydrazinopyridazine), and some related ligands, have been studied using potentiometry, elemental analysis, spectrophotometry and computer simulation. The coordination chemistry of 1-hydrazinophthalazine and the hydrazinopyridazines is similar in that Ca(II), Mg(II), and Mn(II) complexes are not formed, whereas Zn(II), Cu(II) and Fe(II)/Fe(III) complexes are produced. Both kinds of ligand react with Fe(II) to form a brightly coloured tetrazene complex which is insoluble for hydralazine but soluble for prizidilol. Computer simulation studies indicate that the most prevalent metal complex of prizidilol in blood plasma is [Fe2+(Priz−H+]2+ but that this only forms at very high drug concentrations. It is concluded that prizidilol is unlikely to have any direct effects on the metabolism or distribution of the trace elements listed here.
Similar content being viewed by others
References
A. Cole, P.M. May andD.R. Williams,Metal binding by pharmaceuticals. Part III. Copper(II) and zinc(II) interactions with isoniazid, Agents and Actions13, 91–97 (1983).
E.M. Taylor, A.M. Roe andR.A. Slater,SK & F 92657, a novel antihypertensive acting by precapillary vasodilation and β-adrenoreceptor blockade, Clin. Sci.57, 433s-436s (1979).
E.M. Taylor, D. Cameron, R.J. Eden, R. Fielden andD.A. Owen,Hemodynamic profile of a new antihypertensive agent d,l-3-[2-(3-t-butylamino-2-hydroxypropoxy)phenyl]-6-hydrazinopyridazine (SK & F 92657), J. Cardiovasc. Pharm.3, 337–354 (1981).
E.M. Taylor, R.J. Eden, R. Fielden andD.A. Owen,Studies on the automatic nervous system with SK & F 92675, a new antihypertensive agent causing direct arterial vasodilatation and β-adrenoreceptor blockade. J. Cardiovasc. Pharm.3, 355–368 (1981).
A. Bell, M.J. Boyce, W.L. Burland andD.D. Underwood,Assessment of man of SK & F 92657, a novel compound with vasodilator and β-adrenoreceptor blocking activity, Br. J. Clin. Pharmac.9, 299–300P (1980).
J.G. Collier andD.R. Pitcher,Investigation of a combined arteriolar dilator and β-adrenoreceptor antagonist (SK & F 92657) in the peripheral vessels of man. Br. J. Clin. Pharmac.10, 567–571 (1980).
G. Leonetti, L. Terzoli, C. Sala, C. Bianchini andA. Zanchetti,Dose-response curve and time course of the antihypertensive effect of SK & F 92657, a β-receptor-blocking and vasodilating compound, Clin. Sci.59, 461s-463s (1980).
B.E. Karlberg, R. Larsson andK.P. Ohman,Prizidilol (SK & F 92657) in primary hypertension, Clin. Sci.61, 461s-464s (1981).
R. Fariello, C.L. Alicandri, E. Agabiti-Rosesi, G. Romanelli, M. Castellano, M. Beschi, L. Platto, S. Leto de Priolo andG. Muiesan,Effects of prizidilol (SK & F 92657) on blood pressure, haemodynamics, sympathetic nervous system activity and plasma volume in essential hypertension, Clin. Sci.61, 465s-468s (1981).
Introduction to Bio-inorganic Chemistry (Ed.D.R. Williams). C.C. Thomas, Illinois 1976.
S. Fallab andH. Erlenmeyer,Metal ion complexing reactions and biological activity, Helv. chim. ActaXL, 363–368 (1957).
H.M. Perry, Jr. andH.A. Schroeder,Studies on the control of hypertension by hyphex. III. Pharmacological and chemical observations on 1-hydrazinophthalazine, Am. J. med. Sci.228, 396–404 (1954).
P.O. Wester,The urinary excretion of trace elements before and during treatment with hydralazine. Acta med. scand.197, 307–309 (1975).
Th. Kaden andS. Fallab, inAdvances in Chemistry of Coordination Compounds, pp. 654–662 (Ed.Kirschiner). Macmillan, 1961.
D. Walz andS. Fallab,Reactivity of coordination complexes. Parts III and V. The reactions of iron complexes with 1-hydrazinophthalazine. The Fe 2+ catalysed autoxidation of 1-hydrazinophthalazine, Helv. chim. Acta43, 540–543 (1960);44, 13–19 (1961).
S. Fallab,Reactivity of co-ordination compounds, 8: the formation of iron(II) chelates of phthalazine-1-ylhytdrazyl radicals, Helv. chim. Acta45, 1957–1965 (1962).
W.J. Trott, Ph.D. Thesis, University of Wales (1982).
E. Bottari, A.M. Corrie, D.R. Williams et al.,Formation constants for glycinate-proton and-nickel(II) complexes: an investigation of the compatibility of techniques, Annali Chim.68, 813–885 (1978).
A. Sabatini, A. Vacca andP. Gans,MINIQUAD — a general computer program for the computation of formation constants from potentiometric data, Talanta21, 53–77 (1974).
I.G. Sayce,Computer calculation of equilibrium constants of species present in mixtures of metal ions and complexing agents, Talanta15, 1397–1411 (1968).
D.R. Williams,Thermodynamic considerations in co-ordination. Part XIII. Formation constants for the glutaminate- and serinate-proton,-manganese(II),-iron (II),-cobolt(II),-nickel(II),-copper(II), and-zinc(II) systems, J. Chem. Soc., Dalton 1064–1066 (1973).
A.M. Corrie, G.K.R. Makar, M.L.D. Touche andD.R. Williams,Thermodynamic considerations in co-ordination. Part XX. A computerised approach as an alternative to graphical normalised curve fitting as a means of detecting oligonuclear complexes in metal ion-ligand solutions and its application to the zinc(II)- and lead(II)-, and proton-glycine peptide systems, J. Chem. Soc. Dalton 105–110 (1975).
D.D. Perrin andI.G. Sayce,Computer calculations of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta14, 833–842 (1967).
N. Blumenkrantz, A.H. Christiansen andG. Asboe-Hansen,Effect of hydralazine and dihydralazine on connective tissue and binding to serum protein, Scand. J. Rheum.8, 177–183 (1979).
Author information
Authors and Affiliations
Additional information
Part III is reference [1].
Rights and permissions
About this article
Cite this article
Al-Falahi, H., May, P.M., Roe, A.M. et al. Metal binding by pharmaceuticals. Part 4. A comparative investigation of the interaction of metal ions with hydralazine, prizidilol and related compounds. Agents and Actions 14, 113–120 (1984). https://doi.org/10.1007/BF01966843
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01966843