Abstract
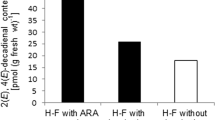
Previous studies conducted in cytosolic extracts of the freshwater hydrozoanHydra vulgaris led to the finding of an abundant 11(R)-lipoxygenase catalyzing the peroxidation of polyunsaturated fatty acid (PUFAs) on the tenth carbon atom from the aliphatic end (ω10 peroxidation). Here we describe experiments aimed at identifying the actual metabolites generated in vivo by such enzymic activity. Homogenates ofH. vulgaris polyps were analyzed by HPLC. This showed the presence of three major components chromatographically identical to three metabolites obtained when incubating the homogenates with exogenous α-linolenic acid (α-LA). The presence, in extracts of polyps prelabelled with [14C]-α-linolenic acid, of radioactive metabolites displaying the same chromatographic properties, substantiated the hypothesis that the natural products isolated in vivo are derived from α-LA. Gas chromatographic analyses revealed that this was the most abundant PUFA in both free and phosphoglyceride-bound fatty acid pools. [1H]-NMR analysis of the endogenous substances, carried out in comparison with products obtained from exogenously incubated α-LA, indicated that their structures were those of 9-hydroxy-, 9-hydroperoxy- and 9-keto-octadeca-10E-12Z-15Z-trienoic acids (9-α-HOTrE,-HPOTrE and-KOTrE).Hydra homogenates transformed 9-α-HPOTrE partly into 9-α-HOTrE and partly into 9-α-KOTrE. Chiral phase HPLC conducted on 9-α-HOTrE established that this metabolite was composed mostly of theR anantiomer. These observations, and the finding that the presence of exogenous arachidonic acid in incubated homogenates significantly reduces the production of α-LA metabolites, provide strong evidence that these compounds are produced by an enzymic activity identical to the previously-describedH. vulgaris (R)-ω10-lipoxygenase. Further experiments suggested that α-LA, acting as the native substrate for this enzyme, is mainly esterified on the 2 position ofHydra phosphoglycerides, and that the production of the α-LA metabolites described here for the first time from natural sources, can be potentially enhanced in vivo by stimuli activating phospholipase A2.
Similar content being viewed by others
References
Pace-Asciak, C. R., and Asotra, S., Free radic. Biol. Med.,7 (1989) 409.
Schewe, T., and Kuhn, H., Trends biochem. Sci.16 (1991) 369.
Yamamoto, S., Free Radic. Biol. Med.10 (1991) 149
Keppler, D., Rev. Physiol. Biochem. Pharmac.121 (1992) 1.
Serhan, C. N., Bioenergetics Biomembranes23 (1991) 105.
De Petrocellis, L., and Di Marzo, V., Prostaglandins Leukotrienes ess. fatty Acids (1994), in press.
Pace-Asciak, C. R., and Martin, J. M., Prostaglandins Leukotrienes Med.16 (1984) 173.
Corey, E. J., Matsuda, S. P. T., Nagata, R., and Cleaver, M. B., Tetrahedron Lett.29 (1988) 2555.
Brash, A. R., Baertschi, S. W., Ingram, C. D., and Harris, T. M., Adv. Prostaglandin Thromboxane Leukotriene Res.19 (1989) 70.
Brash, A. R., Baertschi, S. W., and Harris, T. M., J. biol. Chem.265 (1990) 6705.
Galliard, T., and Phillips, D. R., Biochem. J.124 (1971) 431.
Borgeat, P., Hamberg, M., and Samuelsson, B., J. biol. Chem.251 (1976) 7816.
Hamberg, M., and Samuelsson, B., J. biol. Chem.242 (1967) 5329.
Di Marzo, V., De Petrocellis, L., Gianfrani, C., and Cimino G., Biochem. J.295 (1993) 23.
Di Marzo, V., Gianfrani, C., De Petrocellis, L., Milone, A., and Cimino, G., Biochem. J.,300 (1994), 501.
De Petrocellis, L., Di Marzo, V., and Cimino, G., Experientia49 (1993) 57.
De Petrocellis, L., Di Marzo, V., Gianfrani, C., and Minei, R., Comp. Biochem. Physiol.105C (1993) 219.
Muller, W. A., Leitz, T., Stephan, M., and Lehmann W. D., Roux's Arch. devl. Biol.202 (1993) 70.
Di Marzo, V., Cimino, G., Crispino, A., Minardi, C., Sodano, G., and Spinella, A., Biochem. J.273 (1991) 593.
Brash, A. R., and Hawkins, D. J., Meth. Enzym.187 (1990) 187.
Kato, T., Yamaguchi, Y., Hirano, T., Yokohama, T., Uyehara, T., Namai, T., Tamanaka, S., and Harada, N., Chem. Lett. (1984) 409.
Rama-Rao, A. V., Rajarathnam-Reddy, E., Purandare, A. V., and Varaprasad, C. V. N. S., Tetrahedron43 (1987) 4385.
Fitzpatrick, F. A., and Murphy, R. C., Pharmac. Rev.40 (1989) 229.
Borrelli, L., Carginale, V., Capasso, A., Schneider, T., Leitz, T., De Petrocellis, L., and Di Marzo, V., submitted.
Waite, M., in: Biochemistry of lipids, lipoproteins and membranes, p. 269, Eds D. L. Vance and J. Vance. Elsevier, Amsterdam 1991.
Axelrod, J., Burch, R. M., and Jelsema, C. L., Trends Neurosci.11 (1988) 117.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gianfrani, C., Di Marzo, V., De Petrocellis, L. et al. Hydra vulgaris ω10-lipoxygenase is used in vivo to synthesize new α-linolenic acid metabolites. Experientia 51, 48–56 (1995). https://doi.org/10.1007/BF01964919
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01964919