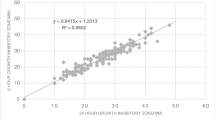
Abstract
The disc diffusion method of susceptibility testing is widely used in diagnostic laboratories throughout the world. It is simple to perform and very suited to the clinical laboratory where a single isolate can be tested with a series of antibiotics in one experiment. For these two reasons the test is by far the commonest type of susceptibility method used. Other methods such as determination of the minimum inhibitory concentration or “breakpoint” methods are used in only a small number of laboratories. If standardisation of methods is to be achieved in order to agree on uniform reporting and guidelines, methods using disc tests are of the highest priority.
Similar content being viewed by others
References
National Committee for Clinical Laboratory Standards Subcommittee on Disk Diffusion Susceptibility Testing: Performance standards for antimicrobial disc susceptibility tests. Approved Standard M2-A3. NCCLS, Villanova, PA, 1984.
World Health Organisation: Standardization of methods for conducting microbic sensitivity tests: second report of the WHO Expert Committee on Antibiotics. WHO Technical Report Series No. 210, Geneva, 1961.
Powell M, Williams JD: Detection of ampicillin-resistantHaemophilus influenzae in United Kingdom laboratories. Journal of Clinical Pathology 1988, 41: 716–719.
Powell M: Antimicrobial resistance inHaemophilus influenzae: epidemiology, mechanisms and therapeutic implications. MD Thesis, University of London, London, 1989.
Moosdeen F, Williams JD, Secker A: Standardization of inoculum size for disc susceptibility testing: a preliminary report of a spectrophotometric method. Journal of Antimicrobial Chemotherapy 1988, 21: 439–443.
Sykes RB, Bonner DP: Monobactam antibiotics: history and development. In: Williams JD, Woods P (ed): Aztreonam: the antibiotic discovery for gram-negative infections. Royal Society of Medicine, London, 1985, p. 1–24.
O'Brien TF, Kent RL, Medeiros AA: Computer-generated plots of results of antimicrobial susceptibility tests. Journal of the American Medical Association 1969, 210: 84–92.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Williams, J.D. Prospects for standardisation of methods and guidelines for disc susceptibility testing. Eur. J. Clin. Microbiol. Infect. Dis. 9, 496–501 (1990). https://doi.org/10.1007/BF01964290
Issue Date:
DOI: https://doi.org/10.1007/BF01964290