Abstract
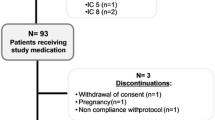
In a prospective study spanning 2 years, 60 patients with grass pollen allergy were treated with either a low dose oral, a high dose oral or a subcutaneous hyposensitization regime. No significant improvement was seen in the orally treated patients whereas those on the subcutaneous hyposensitization regime demonstrated a decreased specific cutaneous reactivity, a rise in specific IgG antibodies and a reduction in symptoms. This study suggests that oral hyposensitization, even with enterosoluble grass pollen capsules, is ineffective.
Similar content being viewed by others
Abbreviations
- CPT:
-
conjunctival provocation test
- SPT:
-
skin prick test
References
Challacombe SJ, Tomasi JB Jr (1980) Systemic tolerance and secretory immunity after oral immunization. J Exp Med 152:1459–1472
Cooper PJ, Carbyshire J, Nunn AJ, Warner JO (1984) A controlled trial of oral hyposensitization in pollen asthma and rhinitis in children. Clin Allergy 7:541–550
Creticos PS, Metre TE van, Mardiney MR, Rosenberg GL, Norman PS, Adkinson NF (1984) Dose response of IgE and IgG antibodies during ragweed immunotherapy. J Allergy Clin Immunol 73:94–104
Curtis HH (1900) The immunizing cure of hay fever. Med News 77:16
Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW (1982) Effect of immunotherapy on immunglobulin E and immunglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Appl Immunol 70:261–271
Golden DBK, Meyers DA, Kagey-Sobotka A, Valentine MD, Lichtenstein MD (1982) Clinical relevance of venom-specific immunglobulin G antibody level during immuntherapy. J Allergy Clin Immunol 69:489–493
Grammer LC, Zeiss CR, Suszko IM, Shaugnessy MA, Patterson R (1982) A double-blind, placebo-controlled trial of polymerized whole ragweed for immunotherapy of ragweed allergy. J Allergy Clin Immunol 69:494–499
Grammer LC, Shaugnessy MA, Suszko IM, Shaugnessy JJ, Patterson R (1983) A double-blind, histamine placebo-controlled trial of polymerized whole grass for immunotherapy of grass allergy. J Allergy Clin Immunol 72:448–453
Hoffmann DR, Gillmann SA, Cummins LH, Kozak PP Jr, Oswald A (1981) Correlation of IgG and IgE antibody level to honcy bee venom allergens with protection to sting challenge. Ann Allergy 46:17–23
Kuhn W, Urbanek R, Forster J, Dreborg S, Burow G (1985) Hyposensibilisierung bei Pollinosis: dreijährige prospektive Vergleichsuntersuchung bei Kindern. Allergologie 8:103–109
Levy DA, Lichtenstein LM, Goldstein EO, Ishizaka K (1971) Immunologic and cellular changes accompanying the therapy of pollen allergy. J Clin Invest 50:360–369
Lichtenstein LM, Norman PS, Winkenwerder WL (1971) A single year of immunotherapy for regweed hay fever. Immunologic and clinical studies. Ann Intern Med 75:663–671
Metzger WJ, Dorminey HC, Richerson HB, Weiler JM, Donnelly A, Moran D (1981) Clinical and immunologic evaluation of glutaraldehyd-modified tyrosine-adsorbed short ragweed extract: a double-blind, placebo-controlled trial. J Allergy Clin Immunol 68:442–448
Möller C, Dreborg S, Lanner A, Björksten B (1986) Oral immunotherapy of children with rhinoconjunctivitis due to birch pollen allergy. Allergy 41:271–279
Mosbech H, Dreborg S, Madsen F, Ohlsson H, Stahl Skov P, Taudorf E, Weeke B (1987) High dose grass pollen tablets used for hyposensitization in hay fever patients. Allergy 42:451–455
Norman PS, Lichtenstein LM, Kagey-Sobotka A, Marsh DG (1982) Controlled evaluation of allergoid in the immunotherapy of ragweed hay fever. J Allergy Clin Immunol 70:248–260
Ortolani C, Pastorello E, Moss RB, Hsu Y-P, Restuccia M, Joppolo G, Miadonna A, Cornelli U, Halpern G, Zanussi C (1984) Grass pollen immunotherapy: A single year double-blind, placebo-controlled study in patients with grass pollen-induced asthma and rhinitis. J Allergy Clin Immunol 73:283–290
Rebien W, Puttonen E, Maasch HJ, Stix E, Wahn U (1982) Clinical and immunological response to oral and subcutaneous immunotherapy with grass pollen extracts. A prospective study. Eur J Pediatr 138:341–344
Taudorf E, Laursen LC, Djurup R, Kappelgaard E, Pedersen CT, Soborg M, Wilkinson P, Weeke B (1985) Oral administration of grass pollen to hay fever patients. Allergy 40:321–335
Taudorf E, Laursen LC, Lanner A, Björksten B, Dreborg S, Soborg M, Weeke B (1987) Oral immunotherapy in birth pollen hay fever. J Allergy Clin Immunol 80:153–161
Urbanek R, Kuhn W, Binder U (1983) Untersuchungen zur Wirksamkeit oraler und parenteraler Hyposensibilisierung mit Pollen-Extrakten. Dtsch Med Wochenschr 38:1433–1437
Urbanek R, Kemeny MD (1988) IgG and IgG subclasses response to dietary antigens in patients with immediate and non-immediate food allergy. Food Allergy, Nestlé Nutrition Workshop Series, vol. 17, Raven Press, New York
Wahn U, Maasch HJ, Geissler W (1984) Leukocyte histamine release and humoral changes during oral and subeutaneous hyposensitization of grass pollen allergic children. Helv Paediatr Acta 39:137–144
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Urbanek, R., Bürgelin, K.H., Kahle, S. et al. Oral immunotherapy with grass pollen in enterosoluble capsules. Eur J Pediatr 149, 545–550 (1990). https://doi.org/10.1007/BF01957689
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01957689