Abstract
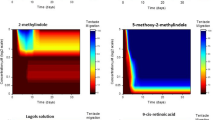
Two pteridine-containing bromophysostigmine alkaloids, urochordamine A and B, which were isolated from ascidians as larval metamorphosis promoters, were converted to more polar compounds, urochordamine A′ and B′, respectively, when left standing in protic solvents. These four compounds promoted larval metamorphosis of the ascidianHalocynthia roretzi in the order A′>A>B>B′, and induced metamorphosis of the pediveliger larvae of the musselMytilus edulis galloprovincialis.
Similar content being viewed by others
References
Pawlik, J. R., in: Ecological Roles of Marine Natural Products, p. 189. Ed. V. J. Paul. Cornell University Press, New York 1992.
Kato, T., Kumanireng, A. S., Ichinose, I., Kitahara, Y., Kakinuma, Y., and Kato, Y., Chem. Lett. (1975) 335.
Kato, T., Kumanireng, A. S., Ichinose, I., Kitahara, Y., Kakinuma, Y., Nishihira, M., and Kato, M., Experientia31 (1975) 433.
Pawlik, J. R., Mar. Biol.91 (1986) 59.
Pawlik, J. R., and Faulkner, D. J., J. exp. mar. Biol. Ecol.102 (1986) 301.
Yvin, J. C., Chevolot, L., Chevolot-Magueur, A. M., and Cochard, J. C., J. nat. Prod.48 (1985) 814.
Kitamura, H., Kitahara, S., and Koh, H. B., Mar. Biol.115 (1993) 387.
Tsukamoto, S., Hirota, H., Kato, H., and Fusetani, N., Tetrahedron Lett.34 (1993) 4819.
Urochordamine A′ (3). UV λmax 350 (log ε 3.60), 339.5 (3.79), 309 (3.75), and 248.5 nm (4.27).1H and13C NMR (DMSO-d 6): Table. FABMS (positive, glycerol matrix)m/z 486/484 (M+H)+, 232 (C11H14N5O)+, and 218 (C10H12N5O)+. HRFAMBS (PEG+NBA matrix)m/z 486.1428, calcd for C22H27 81BrN7O, Δ-1.2mmu).
Ultraviolet spectra were recorded on a Hitachi U-2000 spectrophotometer. Infrared spectra were measured on a JASCO IR Report-100 or IR-700 spectrometer. NMR spectra were recorded on a Bruker ARX-500 NMR spectrometer at 27°C in DMSO-d 6. Residual CHD2SOCD3 (2.49 ppm) and CD3SOCD3 (39.5 ppm) signals were used as internal standards. FAB mass spectra were measured on a JEOL SX 102 mass spectrometer.
Guella, G., Mancini, I., Zibrowius H., and Pietra, F., Helv. chim. Acta72 (1989) 1444 In the aplysinopsin-type compound NHMe and NHMe signals were observed at δ 2.98 (br.d,J=4.8 Hz) and 7.40 (br. q,J=4.8 Hz) in DMSO-d 6, respectively.
Urochordamine B′ (4). UV λmax 351.5 (log ε 3.50), 337.5 (3.57), 324.5 (3.57), and 248.0 nm (4.04).1H NMR (DMSO-d 6) δ: 0.60 (3 H,t, J= 7.0 Hz, 11-H3), 1.57 (1 H,m, 3α-H), 1.84 (2 H, m, 10-H2), 2.24 (1 H, m,3β-H), 2.25 (3 H, br.s, 1-Me), 2.29 (1 H, m, 2α-H), 2.47 (1 H, m, 2β-H), 2.86 (3 H, br. s, 2′-NMe), 3.16 (1 H, t,J=7Hz, 9-H), 3.56 (3 H, s, 1′-Me), 4.73 (1 H, s, 8a-H), 6.30 (1 H, s, 8-H), 6.37 (1 H, s, 7-H), 6.53 (1 H, d,J=8.0 Hz, 5-H), 7.15 (1 H, d,J=8.0 Hz, 4-H), 7.65 (1 H, br.s, 2′-NH), and 8.36 (1 H, s, 6′-H).13C NMR (DMSO-d 6) °: 12.3 (q, C11), 22.1 (t, C10), 28.6 (s, 2′-NMe), 20.0 (s, 1′-Me), 36.4 (s, 1-Me), 37.7 (t, C3), 51.4 (t, C2), 52.1 (d, C9), 60.1 (s, C3a), 85.0 (d, C8a), 109.4 (d, C7), 118.4 (d, C5), 120.1 (s, C6), 126.5 (d, C4), 129.2 (s, C4′a), 132.7 (s, C3b), 146.42 (d, C7′) 146.48 (s, C8′a), 152.3 (s, C6′), 153.3 (s, C7a), 154.6 (s, C2′), and 165.8 (s C4′). HMBC correlations: 4-H/C6 and C7a; 5-H/C3b and C7; 7-H/C3b; 8-H/C3b; 8a-H/C2 and C9; 9-H/C3a, C8a, C6′, and C7′; 10-H2/C9 and C6′; 11-H3/C9 and C10; 7′-H/C6′ and C8′a; 1-Me/C2 and C8a; 1′-Me/C2′; 2-NMe/C2′., FABMS (positive, glycerol matrix)m/z 486/484 (M+H)+, 232 (C11H14N5O)+, and 218 (C10H12N5O)+. HRFABMSm/z 486.1443, calcd for C22H27 81BrN7O, Δ+0.3 mmu).
Composition of artificial seawater: 460 mM NaCl, 10.1 mM KCl, 9.2 mM CaCl2, 35.9 mM MgCl2·6H2O, 17.5 mM MgSO4·7H2O, 10 mM Tris-HCl, pH 8.2.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsukamoto, S., Hirota, H., Kato, H. et al. Effect of urochordamines on larval metamorphosis of ascidians. Experientia 50, 680–683 (1994). https://doi.org/10.1007/BF01952873
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01952873