Abstract
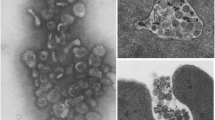
A group of bacterial protein toxins interfere with vesicular trafficking inside cells. Clostridial neurotoxins affect mainly the highly regulated fusion of neurotransmitter- and hormone-containing vesicles with the plasma membrane. They cleave the three SNARE proteins: VAMP, SNAP-25 and syntaxin, and this selective proteolysis results in a blockade of exocytosis. TheHelicobacter pylori cytotoxin is implicated in the pathogenesis of gastroduodenal ulcers. It causes a progressive and extensive vacuolation of cells followed by necrosis, after a cytotoxin-induced alteration of membrane trafficking by late endosomes. Vacuoles originate from this compartment in a rab7-dependent process and swell because they are acidic and accumulate membrane-permeant amines.
Similar content being viewed by others
References
Mims C., Dimmock N., Nash A. and Stephen J. (1995) Mims' Pathogenesis of Infectious Disease, Academic Press, London
Alouf J. E. and Freer J. H., eds (1991) Sourcebook of Bacterial Protein Toxins, Academic Press, London
Menestrina G., Schiavo G. and Montecucco C. (1994) Molecular mechanisms of action of bacterial protein toxins. Mol. Aspects Med.15: 79–193
Simpson L. L., ed. (1989) Botulinum Neurotoxin and Tetanus Toxin, Academic Press, San Diego
Montecucco C., ed. (1995) Clostridial Neurotoxins. Curr. Top. Microbiol. Immunol., vol. 195, Springer, Heidelberg
Montecucco C. and Schiavo G. (1995) Structure and function of tetanus and botulinum neurotoxins. Quart. Rev. Biophys.28: 423–472
Nishiki T., Kamata Y., Nemoto Y., Omori A., Ito T., Takahashi M. et al. (1994) Identification of protein receptor forClostridium botulinum type B neurotoxin in rat brain synaptosomes. J. Biol. Chem.269: 10498–10503
Dolly J. O., Black J., Williams R. S. and Melling J. (1984) Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature307: 457–460
Critchley D. R., Nelson P. G., Habig W. H. and Fishman P. H. (1985) Fate of tetanus toxin bound to the surface of primary neurons in culture: evidence for rapid internalization.J. Cell Biol. 100: 1499–1507
Parton R. G., Ockleford C. D. and Critchley D. R. (1987) A study of the mechanism of internalisation of tetanus toxin by primary mouse spinal cord cultures. J. Neurochem.49: 1057–1068
Wellhoner H. H. (1992) Tetanus and botulinum neurotoxins. In: Handbook of Experimental Pharmacology, vol. 102, pp. 357–417, Herken H. and Hucho F. (eds) Springer-Verlag, Berlin
Schwab M. E. and Thoenen H. (1976) Electron micrscopic evidence for a transsynaptic migration of tetanus toxin in spinal cord motoneurons: an autoradiographic and morphometric study. Brain Res.105: 213–227
Schwab M. E., Suda K. and Thoenen H. (1979) Selective retrograde trans-synaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J. Cell. Biol.82: 798–810.
Williamson, L. C. and Neale E. A. (1994) Bafilomycin A1 inhibits the action of tetanus toxin in spinal cord neurons in cell culture. J. Neurochem.63: 2342–2345
Simpson, L. L., Coffield J. A. and Bakry N. (1994) Inibition of vacuolar adenosine triphosphatase antagonizes the effects of clostridial neurotoxins but not phospholipase A2 neurotoxins. J. Pharmacol. Exp. Ther.269: 155–166
Boquet, P. and Duflot E. (1982) Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc. Natl. Acad. Sci. USA,79: 7614–7618
Boquet P., Duflot E. and Hattecoeur B. (1984) Low pH induces a hydrophobic domain in the tetanus toxin molecule. Eur. J. Biochem.144: 339–344
Roa M. and Boquet P. (1985) Interaction of tetanus toxin with lipid vesicles at low pH. J. Biol. Chem.260: 6827–6835
Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R. and Simpson L. L. (1985) Channels formed by botulinum, tetanus and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc. Natl. Acad. Sci. USA82: 1692–1696
Montecucco C., Schiavo G., Brunner, J., Duflot E., Boquet P. and Roa M. (1986) Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry25:919–924
Gambale F. and Montal M. (1988) Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophys. J.53: 771–783
Montecucco C., Schiavo G. and DasGupta B. R. (1989) Effect of pH on the interaction of botulinum neurotoxins A, B and E with liposomes. Biochem. J.259: 47–53
Menestrina G. Forti S. and Gambale F. (1989) Interaction of tetanus toxin with lipid vesicles: effects of pH, surface charge and transmembrane potential on the kinetics of channel formation. Biophys. J.55: 393–405
Blaustein R. O., Germann W. J., Finkelstein A. and DasGupta B. R. (1989) The N-terminal half of the heavy chain of botulinum type A neurotoxin forms channels in planar phospholipid bilayers. FEBS Lett.226: 115–120
Montal M. S., Blewitt R., Tomich J. M. and Montal M. (1992) Identification of an ion channel-forming motif in the primary structure of tetanus and botulinum neurotoxins. FEBS Lett.313: 12–18
Schmid M. F., Robinson J. P. and DasGupta B. R. (1993) Direct visualization of botulinum neurotoxin-induced channels in phospholipid vesicles. Nature364: 827–830.
Beise J., Hahnen J., Andersen-Beckh B. and Dreyer F. (1994) Pore formation by tetanus toxin, its chain and fragments in neuronal membranes and evaluation of the underlying motifs in the structure of the toxin molecule. Naunyn-Schmiedeberg's Arch. Pharmacol.349: 66–73
Martoglio B., Hofmann M. W., Brunner J. and Dobberstein B. (1995) The protein-conducting channel in the membrane of the endoplasmic reticulum is open laterally toward the lipid bilayer. Cell81: 207–214
Martoglio B. and Dobberstein B. (1996) Snapshots of membrane translocating proteins. Trends Cell Biol.6: 142–147
Driessen A. J. M. (1996) Prokaryotic protein translocation. In: Phoenix D. A. (ed.), Protein Targeting, chap. 5, Portland Press, London
Sollner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S. et al. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature362: 318–324
Ferro-Novick S. and Jahn R. (1994) Vesicle fusion from yeast to man. Nature370: 191–193
Sudhof T. C. (1995) The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature375: 645–653
Rothman J. E. and Wieland F. T. (1996) Protein sorting by transport vesicles. Science222: 227–234
Schiavo G., Poulain B., Rossetto O., Benfenati F., Tauc L. and Montecucco C. (1992) Tetanus toxin is a zinc protein and its inhibition of neurotrasmitter release and protease activity depend on zinc. EMBO J.11: 3577–3583
Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R. et al. (1992) Tetanus and botulinum-B neurotoxins block neurotransmitter release by a proteolytic cleavage of synaptobrevin. Nature359: 832–835
Schiavo G., Shone C. C., Rossetto O., Alexandre F. C. G. and Montecucco C. (1993) Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J. Biol. Chem.268: 11516–11519
Schiavo G., Malizio C., Trimble W. S., Polverino de Laureto P., Milan G., Sugiyama H. et al. (1994) Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala/Ala peptide bond. J. Biol. Chem.269: 20213–20216
Yamasaki S., Hu, Y., Binz T., Kalkuhl A., Kurazono H., Tamura T. et al. (1994) Synaptobrevin/VAMP ofAplysia californica: structure and proteolysis by tetanus and botulinal neurotoxins type D and F. Proc. Natl. Acad. Sci. USA91: 4688–4692
Yamasaki S., Baumeister A., Binz T., Blasi J., Link E. Cornille F. et al. (1994) Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J. Biol. Chem.269: 12764–12772
Rossetto O., Gorza L., Schiavo G., Schiavo N., Scheller R. H. and Montecucco C. (1995) VAMP/Synaptobrevin isoforms 1 and 2 are widely and differentially expressed in nonneuronal tissues. J. Cell Biol.132: 167–179
Mellanby J., Beaumont M. A. and Thompson P. A. (1988) The effect of lantanum on nerve terminals in goldfish muscle after paralysis with tetanus toxin. Neuroscience25: 1095–1106
Neale E. A., Habig W. H., Schrier B. K., Bergey G. K., Bowers L. M. and Koh J. (1989) Application of tetanus toxin for structure-function studies in neuronal cell cultures. In: Eighth International Conference on Tetanus, pp. 66–70, Nisticò G., Bizzini B., Bytchenko B. and Triau R. (eds), Pythagora Press, Rome
Hunt J. M., Bommert K., Charlton M. P., Kistner A., Habermann E., Augustine G. J. et al. (1994) A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron12: 1269–1279
Blasi J., Chapman E. R., Yamasaki S., Binz T., Niemann H. and Jahn R. (1993) Botulinum neurotoxin C blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J.12: 4821–4828
Schiavo G., Shone C. C., Bennett M. K., Scheller R. H. and Montecucco C. (1995) Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J. Biol. Chem.270: 10566–10570
Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., DeCamilli P., Südhof T. C. et al. (1993) Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature365: 160–163
Binz T., Blasi J., Yamasaki S., Baumeister A., Link E., Südhof T. C. et al. (1994) Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem.269: 1617–1620
Schiavo G., Rossetto O., Catsicas S., Polyverino de Laureto P., DasGupta B. R., Benfenati F. et al. (1993) Identification of the nerve-terminal targets of botulinum neurotoxins serotypes A, D and E. J. Biol. Chem.268: 23784–23787
Schiavo G., Santucci A., DasGupta B. R., Metha P. P., Jontes J., Benfenati F. et al. (1993) Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett.335: 99–103
Osen-Sand A., Staple J. K., Naldi E., Schiavo G., Rossetto O., Petitpierre S. et al. (1996) Common and distinct fusionproteins in axonal growth and transmitter release. J. Comp. Neurol.367: 222–234
Foran P., Lawrence G. W., Shone C. C., Foster K. A. and Dolly J. O. (1996) Botulinum neurotoxin C1 cleaves both syntaxin and SNAP25 in intact and permeabilized chromaffin cells: correlation with its blockade of catecholamine release. Biochemistry35: 2630–2636
Williamson L. C., Halpern J. L., Montecucco C., Brown J. E. and Neale E. A. (1996) Clostridial neurotoxins and substrate proteolysis in intact neurons: botulinum neurotoxin C acts on SNAP-25. J. Biol. Chem.271: 7694–7699
Gansel M., Penner R. and Dreyer F. (1987) Distinct sites of action of clostridial neurotoxins revealed by double poisoning of mouse motor-nerve terminals. Pflugers Arch.409: 533–539
Minton N. (1995) Molecular genetics of clostridial neurotoxins. Curr. Top. Microbiol. Immunol.195: 161–194
Lebeda F. J. and Olson M. A. (1994) Secondary structural predictions for the clostridial neurotoxins. Proteins: Structure, Function & Genetics20: 293–300
Rossetto O., Schiavo G., Montecucco C., Poulain B., Deloye F., Lozzi L. et al. (1994) SNARE motif and neurotoxin recognition. Nature372: 415–416
Montecucco C. and Schiavo G. (1993) Tetanus and botulism neurotoxins: a new group of zinc proteases. Trends Biochem. Sci.18: 324–327
Witcome M., Rossetto O., Montecucco C. and Shone C. C. (1996) Substrate residues distal to the cleavage site of botulinum type B neurotoxin play a role in determining the specificity of its endopeptidase activity. FEBS Lett.386: 133–136
Pellizzari R., Rossetto, O., Lozzi, L., Giovedi S., Johnson E., Shone C. C. et al., (1996) Structural determinants of the specificity for VAMP/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J. Biol. Chem.271: 20353–20358
Shone C. C., Quinn, C. P., Wait R., Hallis B., Fooks S. G. and Hambleton, P. (1993) Proteolytic cleavage of synthetic fragments of vescile-associated membrane protein isoform-2 by botulinum type B neurotoxin. Eur. J. Biochem.217: 965–971
Grote E., Hao J. C., Bennett M. K. and Kelly R. B. (1995) A targeting signal in VAMP regulating transport to synaptic vesicle. Cell81: 581–589
Desnos, C., Clift-O'Grady L. and Kelly, R. B. (1995) Biogenesis of synaptic vesicles in vitro. J. Cell. Biol.130: 1041–1049
Marshall B. J. and Warren J. R. (1984) Unidentified curved bacilli in the stomach of patients with gastric and peptic ulceration. Lanceti: 1311–1315
Marshall B. J., Armstrong J. A., McGechie D. B. and Glancy R. J. (1985) Attempt to fulfil Koch's postulates for pyloric campylobacter Med. J. Austr.142: 436–439
Eurogast Study Group (1993) An international association betweenHelicobacter pylori infection and gastric cancer. Lancet341: 1359–1362
Parsonnet J., Hansen S., Rodriguez L., Gelb A., Warnke A., Jellum E. et al. (1994)Helicobacter pylori infection and gastric lymphoma. New Engl. J. Med.330: 1267–1271
Cover T. L. and Blaser, M. J. (1993)Helicobacter pylori and gastroduodenal disease. Annu. Rev. Med.43: 135–145
Telford, J. L., Covacci A., Ghiara P., Montecucco C. and Rappuoli R. (1994) Unravelling the pathogenic role ofHelicobacter pylori in peptic ulcer: potential new therapies and vaccines. Trends Biotechnol.12: 420–426
Blaser M. J. (1996) The bacteria behind ulcers. Sci. Am.274: 104–107
Leunk R. D., Johnson P. T., David B. C., Kraft W. G. and Morgan D. R. (1988) Cytotoxic activity in broth-culture filtrates ofCampylobacter pylori. J. Med. Microbiol.26: 93–99
Cover T. L. and Blaser M. J. (1992) Purification and characterization of the vacuolating toxin fromHelicobacter pylori. J. Biol. Chem.287: 10570–10575
Telford, J. L., Ghiara P., Dell'Orco, M., Comanducci M., Burroni D., Bugnoli M. et al. (1994) Gene structure of theHelicobacter pylori cytotoxin and evidence of its key role in gastric disease. J. Exp. Med.179: 1653–1658
Schmitt W., and Haas R. (1994) Genetic analysis of theHelicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol. Microbiol.12: 307–319
Phadnis S. H., Ilver D., Janzon L., Normark S. and Westblom T. U. (1994) Pathological significance and molecular characterization of the vacuolating toxin gene ofHelicobacter pylori. Infect. Immun.62: 1557–1565
Cover T. L., Tummuru M. K. R., Cao P., Thompson S. A. and Blaser M. J. (1994) Divergence of genetic sequences for the vacuolating cytotoxin amongHelicobacter pylori strains J. Biol. Chem.269: 10566–10573
Marchetti M., Aricò B., Burroni D., Figura N., Rappuoli R. and Ghiara P. (1995)Helicobacter pylori infection in a mouse model that mimics human disease. Science267: 1655–1658
Tompkins L. S. and Falkow S. (1995) The new path to preventing ulcers. Science267: 1621–1622
Moll G., Papini E., Colonna R., Burroni D., Telford J., Rappuoli R. et al. (1996) Lipid interaction of the 37 kDa and 58 kDa fragments of theHelicobacter pylori cytotoxin. Eur. J. Biochem.234: 947–952.
Lupetti P., Heuser J. E., Manetti R., Massari P., Lanzavecchia S., Bellon P. L. et al. (1996) Oligomeric and subunit structure of theHelicobacter pylori vacuolating cytotoxin. J. Cell Biol.133: 801–807
de Bernard M., Papini E., De Filippis E., Gottardi E., Telford J., Manetti M. et al. (1995) Low pH activates the vacuolating toxin ofHelicobacter pylori which becomes acid and pepsin resistant. J. Biol. Chem.270: 23937–23940
Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., et al. (1989) Cytotoxin production byCampylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J. Clin. Microbiol.27: 225–226
Cover T. L., Puryear W., Perez-Perez G. I. and Blaser M. J. (1991) Effect of urease of HeLa cell vacuolation induced byHelicobacter pylori vacuolating cytotoxin. Infect. Immun.59: 1264–1270
Papini E., Bugnoli M., de Bernard M., Figura N., Rappuoli R. and Montecucco C. (1993) Bafilomycin A1 inhibitsHelicobacter pylori induced vacuolization of HeLa cells. Mol. Microbiol.7: 323–327
Papini E., Gottardi E., Satin B., de Bernard M., Telford J., Massari P. et al. (1996) The vacuolar ATPase proton pump is present on intracellular vacuoles induced byHelicobacter pylori. J. Med. Microbiol.44: 1–6
Papini E., De Bernard M., Milia E., Bugnoli M., Zerial M., Rappuoli R. et al. (1994) Cellular vacuoles induced byHelicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. USA91: 9720–9724
Chavrier P., Parton R. G., Hauri H. P., Simons K. and Zerial M. (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell62: 317–329
Nuoffer C. and Balch W. E. (1994) GTPases: multifunctional molecular switches regulating vesicular traffic. Annu. Rev. Biochem.63: 949–990
Pfeffer S. R., Dirac-Svejstrup B. and Soldati T. (1995) Rab GDP dissociation inhibitor: putting Rab GTPases in the right place. J. Biol. Chem.270: 17057–17059
Simons K. and Zerial M. (1993) Rab proteins and the road maps for intracellular transport. Neuron11: 789–799
Zerial M. and Huber L. (1996) Guidebook to the Small GTPases, Oxford University Press, Oxford.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Montecucco, C., Papini, E. & Schiavo, G. Bacterial protein toxins and cell vesicle trafficking. Experientia 52, 1026–1032 (1996). https://doi.org/10.1007/BF01952098
Issue Date:
DOI: https://doi.org/10.1007/BF01952098