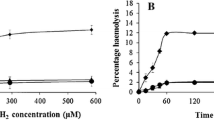
Summary
Invertebrates possess lytic molecules which lyse vertebrate erythrocytes. In all the species studied so far, hemolytic activity depends on proteins which possess a wide range of reactivity. It is generally calcium-dependent and heat-labile, although calcium-independent and heat-stable hemolysins have also been detected. The molecules interact with sugars or lipids which could represent the membrane receptors by which circular lesions on target membranes are produced.
On the basis of some analogies with vertebrate lytic molecules it is conceivable that the hemolysins evolved from a common ancestral gene which also led to vertebrate pore-forming proteins.
Similar content being viewed by others
References
Anderson, R. S., Day, N. K. B., and Good, R. A., Specific hemagglutinin and a modulator of complement in cockroach hemolymph. Inf. Immun.5 (1972) 55–59.
Anderson, R. S., Hemolysin and hemagglutinins in the coelomic fluid of a polychaete annelid,Glycera dibranchiata. Biol. Bull.159 (1980) 259–268.
Anderson, R. S., and Chain, B. M., Antibacterial activity in the coelomic fluid of a marine annelid,Glycera dibranchiata. J. Invertebr. Pathol.40 (1982) 320–326.
Bernheimer, A. W., and Avigard, L. S., Properties of a toxin from the sea anemoneStoichactis helianthus including specific binding to sphingomyelin. Proc. natl Acad. Sci. USA73 (1976) 467–471.
Bertheussen, K., The cytotoxic reaction in allogenic mixtures of Echinoid phagocytes. Exp. Cell. Res.120 (1979) 373–381.
Bertheussen, K., Complement-like activity in sea urchin coelomic fluid. Devl comp. Immun.7 (1983) 21–28.
Bertheussen, K., Complement and lysins in invertebrates. Devl comp. Immun.3 (1984) 173–181.
Bretting, H., and Renwrantz, L., Untersuchungen von Invertebraten des Mittelmeeres auf ihren Gehalt an hämagglutinierenden Substanzen. Z. Immun. Forsch.145 (1973) 242–249.
Canicatti, C., and Parrinello, N., Chromatographic separation of coelomic fluid fromHolothuria polii (Echinodermata) and partial characterization of the fractions reacting with erythrocytes. Experientia39 (1983) 764–766.
Canicatti, C., and Parrinello, N., Hemagglutinin and hemolysin levels in the coelomic fluid fromHolothuria polii (Echinodermata) following sheep erythrocyte injection. Biol. Bull.168 (1985) 175–182.
Canicatti, C., and Ciulla, D., Studies onHolothuria polii (Echinodermata) coelomocyte lysate. I. Hemolytic activity of coelomocyte hemolysins. Devl comp. Immun.11 (1987) 705–712.
Canicatti, C., Evolution of the lytic system in Echinoderms. I. Naturally occurring hemolytic activity inParacentrotus lividus (Echinoidea) coelomic fluid. Boll. Zool.54 (1987) 325–329.
Canicatti, C., Parrinello, N., and Arizza, V., Inhibitory activity of sphingomyelin on hemolytic activity of coelomic fluid ofHolothuria polii (Echinodermata). Devl comp. Immun.11 (1987) 29–35.
Canicatti, C., Membrane damage by coelomic fluid fromHolothuria polii (Echinodermata). Experientia43 (1987) 611–614.
Canicatti, C., and D'Ancona, G., Cellular aspects ofHolothuria polii immune response. J. Invertebr. Path.53 (1988) 152–158.
Canicatti, C., and Ciulla, D., Studies onHolothuria polii (Echinodermata) coelomocyte lysate. II. Isolation of coelomocyte hemolysins. Devl comp. Immun.12 (1988) 55–64.
Canicatti, C., and Grasso, M., Biodepressive effect of zinc on humoral effector ofHolothuria polii immune response. Mar. Biol.99 (1988) 393–396.
Canicatti, C., Ciulla, D., and Farina-Lipari, E., The hemolysin producer coelomocytes. Devl comp. Immun.12 (1988) 729–736.
Canicatti, C., The lytic system ofHolothuria polii (Echinodermata). A review. Boll. Zool.55 (1988) 139–144.
Canicatti, C., Evolution of lytic system in Echinoderms. II. Naturally-occurring hemolytic activity inMarthasterias glacialis (Asteroidea). Comp. Biochem. Physiol.93A (1989) 587–591.
Canicatti, C., Protease activity inHolothuria polii coelomic fluid and coelomocyte lysate. Comp. Biochem. Physiol. (1989) in press.
Cenini, P., Comparative studies on haemagglutinins and haemolysins in an annelid and a primitive crustacean. Devl comp. Immun.7 (1983) 637–640.
Cheng, T. C., and Rodrick, G. E., Identification and characterization of lysozymes from the hemolymph of the salt shelled clamMya arenaria. Biol. Bull.147 (1974) 311–320.
Cheng, T. C., Chorney, M. J., and Yoshino, T. P., Lysozyme-like activity in the hemolymph ofBiomphalasie glabrata challenged with bacteria. J. Invertebr. Path.29 (1977) 170–174.
Cruz, O. J. M., and Day, N. K., Structural and functional similarities between the major hemolymph protein of fall armyworm and cat C4 binding protein of the complement system. Devl comp. Immun.9 (1985) 541–550.
Day, N. K. B., Gewzy, H., Johannsen, R., Finstad, J., and Good, R. A., Complement and complement-like activity in lower vertebrates and invertebrates. J. exp. Med.132 (1970) 941–951.
Day, N. K. B., Geiger, H., Finstad, J., and Good, R. A., A starfish hemolymph factor which activates vertebrate complement in the presence of Cobra venom factor. J. Immun.109 (1972) 164–167.
Dourmashkin, R. R., Deteix, P., Simone, C. B., and Henkart, P. A., Electron microscopic demonstration of lesions in target cell membranes associated with antibody dependent cellular cytotoxicity. Clin. exp. Immun.42 (1980) 554–563.
Du Pasquier, L., and Duprat, P., Aspects humoreaux et cellulaires d'une immunité naturelle non spécifique chez l'oligochèteEisenia foetida (Lumbricidae). C.r. Acad. Sci. Paris266 (1968) 538–546.
Fusetani, N., Yasukawa, K., Matsunaga, W., and Hashimoto, K., Isolation and identification of three hemolysins from the hydroidSolanderia secunda (Inaba). Comp. Biochem. Physiol.83B (1986) 511–513.
Goldfarb, R. H., Role of proteases in NK activity, in: Mechanisms of Cytotoxicity by NK Cells, pp. 205–212. Ed. R. Haberman. Academic Press, London 1985.
Hültmark, O., Steiner, H., Rasmusson, T., and Boman, H. G., Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pulpae ofHyalophora cecropia. Eur. J. Biochem.106 (1980) 7–16.
Janatova, J., Lorenz, P. W., Schechter, A. N., Prahl, J. W., and Tack, B. R., Third component of human complement. Appearance of a sulphydryl group following chemical or enzymatic inactivation. Biochemistry19 (1980) 4471–4483.
Kamiya, H., Muramoto, K., and Goto, R., Cytotoxicity and hemolysis by an acqueous extract of a marine sponge,Clathria sp., Symp. Natural Toxins, China 1985.
Kauschke, E., and Mohring, W., Comparative analysis of hemolytic and hemagglutinating activities in the coelomic fluid ofEisenia foetida andLumbricus terrestris (Annelida, Lumbricidae). Devl comp. Immun.11 (1987) 331–341.
Koch, C., and Nielsen, H. E., Activation of vertebrate complement byHelix pomatia haemolymph. Devl comp. Immun.8 (1984) 15–22.
Laulan, A., Lestage, J., Chateaureynand-Duprat, E., and Fontaine, M., Mise en évidence de substances contenues dans le liquide coélomique deLumbricus terrestris, possédant des fonctions communes avec celles de certains composants du complément humain. Annls Immun.134 (1983) 223–241.
Leippe, M., and Renwrantz, J., Release of cytotoxic and agglutinating molecules by Mytilus hemocytes. Devl comp. Immun.12 (1988) 297–308.
Masson, D., and Tschopp, J., Isolation of a lytic pore-forming protein (perforin) from cytolytic T-lymphocytes. J. biol. Chem.260 (1985) 9069.
Masson, D., Nabholz, M., Estrade, C., and Tschopp, J., Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J.5 (1986) 1595–1600.
Masson, D., and Tschopp, J., A family of serine esterases in lytic granules of cytolytic T-lymphocytes. Cell49 (1987) 679–685.
Michaels, D. W., Membrane damage by a toxin from the sea anemoneStoichactis helianthus. I. Formation of transmembrane channels in lipid bilayers. Biochim. biophys. Acta555 (1979) 67–78.
Müller-Eberhard, H. J., and Miescher, P. A., Complement. Springer Verlag, Berlin 1985.
Nigrelli, R. F., Stempien, M. F., Ruggieri, G. D., Liguori, U. R., and Cecil, J. T., Substances of potential biomedical importance from marine organisms. Fed Proc.26 (1967) 1197–1205.
Norton, R. S., Bobek, G., Thomson, M., Moritz, R. L., and Simpson, R. S., Proteins with cardiac stimulatory and hemolytic activity from the sea anemoneActinia tenebrosa. Toxicon7 (1989) 66–67.
Pangburn, M. K., and Müller-Eberhard, H. J., Relation of a putative thioester bound in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J. exp. Med.152 (1980) 1102–1112.
Parrinello, N., Rindone, D., and Canicatti, C., Naturally occurring hemolysins in the coelomic fluid ofHolothuria polii Delle Chiaje (Echinodermata). Devl comp. Immun.3 (1979) 45–54.
Parrinello, N., and Rindone, D., Studies on the natural hemolytic system of the annelid wormSpirographis spallanzanii viviani (Polychaeta). Devl comp. Immun.5 (1981) 33–42.
Phipps, D., Menger, M., Chadwich, J. S., and Aston, W. P., A cobra venom factor (CVF)-induced C3 convertase activity in the hemolymph ofGallesia mellonella. Devl comp. Immun.11 (1987) 37–47.
Platts-Mill, T. A., and Ishizaka, K., Activation of the alternative pathway of human complement by rabbit cells. J. Immun.113 (1974) 348–358.
Podack, E. R., and Tschopp, J., Circular polymerization of the ninth component of complement. J. biol. Chem.257 (1982) 15204–15212.
Podack, E. R., Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. Nature302 (1983) 442–445.
Podack, E. R., Young, J. D. E., and Cohn, Z. A., Isolation and biochemical functional characterization of perforins from cytolytic T-cell granules. Proc. natl Acad. Sci. USA82 (1985) 8629–8638.
Reich, E., Rifkin, D. B., and Shaw, E., Protease and biological control. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 1975.
Roch, P., Protein analysis of earthworm coelomic fluid. I. Polymorphic system of the natural hemolysins ofEisenia foetida Andrei. Devl comp. Immun.3 (1979) 588–608.
Roch, P., Valembois, P., Davant, N., and Lassegues, M., Protein analysis of carthworm coelomic fluid, II. Isolation and biochemical characterization of theEisenia foetida Andrei factor (EFAF). Comp. Biochem. Physiol.698 (1981) 829–836.
Roch, P., Davant, N., and Lassegues, M., Isolation of agglutinins and lysins form earthworm coelomic fluid by gel filtration followed by chromatofocusing. J. Chromat.290 (1984) 231–235.
Roch, P., Canicatti, C., and Valembois, P., Interaction between the hemolytic system of the earthwormEisenia foetida Andrei and the SRBC membrane. Biochim. biophys. Acta983 (1989) 193–198.
Ryoyama, K., Studies on the biological properties of coelomic fluid of sea urchin. I. Naturally occurring hemolysin in sea urchin. Biochim. biophys. Acta320 (1973) 157–165.
Tack, B. R., Harrison, R. A., Janatova, J., Thomas, M. L., and Prahl, J. W., Evidence for presence of an internal thioester bound in third component of human complement. Proc. natl Acad. Sci.77 (1980) 5464–5472.
Tschopp, J., Circular polymerization of the membranolytic ninth component of complement. J. Biol. Chem.259 (1984) 10569–10573.
Tschopp, J., Masson, D., and Stanley, K. K., Structural/function similarity between proteins involved in complement and cytotoxic T-lymphocyte-mediated cytolysis. Nature322 (1986) 831–834.
Tschopp, J., Masson, D., and Schafer, S., Inhibition of the lytic activity of perforin by lipoproteins. J. Immun.137 (1986) 1950–1953.
Tschopp, J., Masson, D., Schafer, S., Peitsch, M., and Preissner, K. T., The heparin binding domain of S/protein/Vitronectin binds to complement components C7, C8 and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Biochemistry27 (1988) 4103–4109.
Tuan, T. L., and Yoshino, T. P., Natural hemolysin in the hemolymph of freshwater bivalveCorbicula fluminea. Devl comp. Immun. suppl 3 (1984) 193.
Tuckova, L., Rejnek, J., Sima, P., and Ondiejova, R., Lytic activities in coelomic fluid ofEisenia foetida andLumbricus terrestris. Devl comp. Immun.10 (1986) 181–189.
Valembois, P., Roch, P., Lassegues, M., and Cassand, P., Antibacterial activity of the hemolytic system from the earthwormEisenia foetida Andrei. J. Invertebr. Path.40 (1982) 21–27.
Valembois, P., Lassegues, M., Roch, P., and Vaillier, J., Scanning electron microscopic study of the involvement of coelomic cells in earthworm antibacterial defence. Cell Tissue Res.240 (1985) 479–484.
Valembois, P., Roch, P., and Lassegues, M., Antibacterial molecules in Annelids, in: Immunity in Invertebrates, pp. 14–93. Ed. M. Brehelin. Springer Verlag, Berlin (1986).
Weinheimer, P. F., Evans, E. E., Stround, R. M., Acton, R. T., and Painter, B., Comparative immunology: Natural hemolytic system of the spiny lobster,Panulirus arguus. Proc. Soc. exp. Biol. Med.130 (1969) 322–326.
Weinheimer, P. F., Acton, R. T., Cushing, J. E., and Evans, E. E., Reactions of sipunculid fluid with erythrocytes. Life Sci.9 (1970) 145–151.
Yoshino, T. P., and Tuan, T. L., Soluble mediators of cytolytic activity in hemocytes of the asian clamCorbicula fluminea. Devl comp. Immun.9 (1985) 515–522.
Young, J. D. E., Cohn, Z. A., and Podack, E. R., The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T-cells: Structural, immunological and functional similarities. Science233 (1986) 184–190.
Ziccardi, R. J., The three components of human complement (C1): Activation and control, in: Complement, pp. 167–184. Eds H. J. Müller-Eberhard and P. A. Miescher. Springer Verlag, Berlin 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Canicatti, C. Hemolysins: Pore-forming proteins in invertebrates. Experientia 46, 239–244 (1990). https://doi.org/10.1007/BF01951753
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01951753